Science > Chemistry > Third Row Elements > Hydroxides of Third Row Elements
In this article, we shall study hydroxides of third row elements.
Different types of hydroxides of third-row elements are classified according to their mode of dissociation. According to Arrhenius’s theory, the acid is the substance which gives H+ ions in an aqueous medium, while the base is the substance which gives OH– ions in an aqueous medium.
Let us use M-O-H be the general formula to represent a hydroxy compound of third row elements. The mode of ionization decides the nature of hydroxide whether it is acidic or basic.
Types of Hydroxy Compounds:
Basic hydroxy compound:
The hydroxy compounds which give OH– ions in an aqueous medium are called basic hydroxides.
M — O — H (aq.) → M+ + OH–
This is possible when the element has very low ionization potential. Valence electrons are loosely held by the atom. Due to very low ionization enthalpy and electronegativity metal atom cannot hold valence electrons. Electron pair between M and O is pulled more towards more electronegative oxygen. M-O bond becomes weak while the O-H bond becomes strong. Thus the bond between M and OH breaks
e.g. hydroxide NaOH of sodium and hydroxide Mg(OH)2 of magnesium are basic hydroxy compounds.
NaOH (aq.) → Na + + OH–
Mg(OH) 2 (aq.) → Mg 2 + + 2 OH –
Both hydroxides give OH– ions in aqueous medium.
Acidic Hydroxy Compounds (Oxyacids):
The hydroxy compounds which give OH – ions in an aqueous medium are called basic hydroxides.
M — O — H (aq.) → MO – + H +
This is possible when the element has greater ionization potential. Valence electrons are strongly held by atom. Due to higher ionization enthalpy and electronegativity, metal atom hold valency electrons strongly. Electron pair between M and O is pulled more towards more electronegative element M. As a result of which bond M-O-H behaves as a base.
M-O bond becomes strong while the O-H bond becomes weak. Thus the bond between MO and H breaks, which results in the production of H + ions in aqueous medium.
The hydroxy compounds which give H + ions in an aqueous medium are called acidic hydroxy compounds or oxyacids. If M-O-H is assumed oxyacid then O-H bond break in an aqueous medium and it will give H + ions.
e.g. Si(OH)4 of Silicon, P (OH)3 and PO (OH)3 of Phosphorous, SO (OH)2 and SO2(OH)2 of sulphur , ClOH, ClO(OH) , ClO2(OH) and ClO3(OH) of chlorine are acidic hydroxy compounds.
Amphoteric Hydroxy Compound:
Hydroxy compound which acts as acid as well as base and can neutralize the acid, as well as base producing salt and water, is called amphoteric hydroxide.
Al(OH)3 of aluminium is an amphoteric oxide. It neutralizes acid as well as base producing a salt and water.
Al (OH)3 (as a base) + 3HCl → AlCl 3 + 3 H2O
Al(OH)3 (as an acid) + NaOH → NaAlO2 (Sodium meta-aluminate) +2 H2O
Thus the nature of hydroxides or oxyacids is mainly governed by the ionization potential of elements.
Trends in acid -base behaviour of hydroxy compounds:
It is seen that as we move from Na to Cl along the third row, the basic character of hydroxy compounds gradually decreases while acidic character gradually increases.
The trend in acid-base behaviour of hydroxy compounds of the third row can be summarized as follows.
| Elements | Na | Mg | Al | Si | P | S | Cl |
| Hydroxy Compounds | NaOH | Mg(OH)2 | Al(OH)3 | Si(OH)4 | PO(OH)3 | SO2(OH)2 | ClO3(OH) |
| Acidic or Basic strengths | Very strongly basic | Strongly basic | Amphoteric | Very weakly acidic | Weakly acidic | Strongly acidic | Very strongly acidic |
Explanation:
The nature of the hydroxy compound mainly depends upon the ionization potential of elements. The trend is so because along third-row ionization potential increase, electronegative character increases, atomic size decreases. Along the third row difference in electronegativity of the element M and that of Oxygen decreases.
If ionization potential of elements is low, then such hydroxy compound give OH – ions in an aqueous medium and hence is basic in nature.
M — O — H (aq) → M + + OH –
NaOH and Mg(OH)2 are basic. Na and Mg have low ionization potential. Na – O and Mg – O bonds are weaker than O-H bond. And thus the bond between M and O breaks to give OH– ions.
NaOH (aq.) → Na + + OH–
Mg(OH) 2 (aq.) → Mg 2 + + 2 OH –
If ionization potential of an element is greater, then such hydroxy compound gives H + ions in an aqueous medium and hence is acidic in nature.
M — O — H (aq) → M O– + H +
Si(OH)4 of Silicon, P(OH)3 and PO(OH3 of Phosphorous, SO(OH)2 and SO2(OH)2 of sulphur, ClOH, ClO(OH), ClO2(OH) and ClO3(OH) of chlorine are acidic hydroxy compounds.
Al(OH)3 is amphoteric. It neutralizes acid as well as base producing salt and water. Hence it exhibits both the properties hence it is an amphoteric oxide.
Scientific Reasons:
Hydroxy compound of Sodium NaOH is a strong base.
The hydroxy compounds which give OH – ions in an aqueous medium are called basic hydroxides.
M — O — H (aq) → M + + OH –
This is possible when the element has very low ionization potential. Valency electrons are loosely held by atom. Due to very low ionization potential and electronegativity, metal atom cannot hold valency electrons. Electron pair between M and O is pulled more towards more electronegative oxygen. M-O bond becomes weak while the O-H bond becomes strong.
Sodium is the strongest electropositive element. Na has lower ionization potential and lower electronegativity. Na -O bond breaks more readily in an aqueous medium and Sodium hydroxide ionizes as
NaOH (aq.) → Na + + OH–
Mg (OH)2 is weakly basic than NaOH.
The hydroxy compounds which give OH – ions in an aqueous medium are called basic hydroxides.
M — O — H (aq) → M + + OH –
This is possible when the element has very low ionisation potential. Valency electrons are loosely held by atom. Due to very low ionisation potential and electronegatively metal atom cannot hold valency electrons. Electron pair between M and O is Pulled more towards more electronegative oxygen. M-O bond becomes weak while the O-H bond becomes strong.
Na has lower ionisation potential and lower electronegativity than that of Mg. So Na – O bond is relatively weak than Mg – O bond. Na -O bond breaks more readily than the Mg-O bond in an aqueous medium. Thus Mg(OH)2 is weakly basic than NaOH.
Aluminium hydroxide is amphoteric compound.
Hydroxy compound which acts as acid as well as base and can neutralise acid, as well as base producing salt and water, is called amphoteric hydroxide.
Al(OH) 3 of aluminium is an amphoteric oxide. It neutralises acid as well as base producing a salt and water.
Al (OH)3 (as a base) + 3HCl → AlCl 3 + 3 H2O
Al(OH)3 (as an acid) + NaOH → NaAlO2 (Sodium meta-aluminate) + 2 H2O
It acts as a base when treated with strong acid. It acts as an acid when treated with a strong base. Due to its dual character, Al(OH)3 is amphoteric in nature.
Atomic size of Aluminium is smaller than Sodium and Magnesium and larger than Silicon, Phosphorous, Sulphur and Chlorine. The ionisation potential of Aluminium is larger than Sodium and Magnesium and lesser than Silicon, Phosphorous, Sulphur and Chlorine. Electronegativity of Aluminium is larger than Sodium and Magnesium and lesser than Silicon, Phosphorous, Sulphur and Chlorine. Thus in aluminium hydroxide, both Al – O and O – H bonds have equal strength. Hence the fission of bond depends on the attacking reagent. Hence Aluminium hydroxide is amphoteric compound.
Orthosilicic acid Si(OH)4 is very weak acid.
Electronegativity of Silicon is 1.8 units. The ionisation potential of Silicon is higher than that of Aluminium.
Si – O is covalent bond with the ionic character. Hence Si – O bond is a strong bond. Oxygen atom pulls the shared electron in O – H bond towards itself and on fission produces H+ ions. However, O-H bond is not broken in water.
The orthosilicic acid reacts with strong alkali on heating.
H4SiO4 + 2 NaOH → Na2Si 3 + 3 H2O
Hydroxy compounds of Phosphorous, Sulphur And Chlorine are acidic.
Phosphorous, Sulphur And Chlorine are non-metals with smaller atomic size, high nuclear charge, High ionisation potential and high electronegativity. These elements have very little or practically no tendency to give an electron to Oxygen.
In the structure of M – O – H Oxygen, therefore, tries to pull electron pair between O – H towards itself, This releases H+ ions in aqueous solution.
Structures of Hydroxy Compounds of Phosphorous or Oxyacids of Phosphorous:
Phosphorous acid (P(OH)3 OR H3PO3) and Phosphoric acid (PO(OH)3 OR (H3PO4)
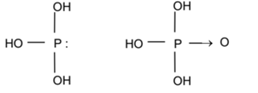
H3PO3 + 2 NaOH → Na2HPO3 + 2 H2O
H3PO4 + 3 NaOH → Na3PO4 + 3 H2O
Phosphoric acid has an un-hydrogenated oxygen atom so O-H bond breaks readily. Hence phosphorous acid is stronger than phosphorous acid.
Structures of Hydroxy compounds of Sulphur or Oxyacids of Sulphur:
Sulphurous acid SO(OH)2 OR (H2SO3) and Sulphuric acid SO2(OH)2 OR (H2SO4)
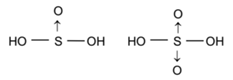
H2SO3 + 2 NaOH → Na2SO3 + 2 H2O
H2SO4 + 2 NaOH → Na2SO4 + 2 H2O
These oxyacids of sulphur are strongly acidic due to greater IP of sulphur. Sulphuric acid has two un-hydrogenated oxygen atoms while in sulphurous acid there has one un-hydrogenated oxygen atom hence, sulphuric acid is stronger than sulphurous acid.
” Greater the Oxidation no. More the Acidic Nature”. In H2SO4 the oxidation no. of the central atom Sulphur is +6 and that in H2SO3 is +4. The oxidation number of sulphur is greater in sulphuric acid than the sulphurous acid. Hence sulphuric acid is stronger than sulphurous acid.
Hydroxy compounds of Chlorine or Oxyacids of Chlorine:
Hypochlorous acid Cl(OH) OR (HOCl), Chloric acid ClO2(OH) OR (HClO3), Perchloric acid ClO3(OH) OR (HClO4)
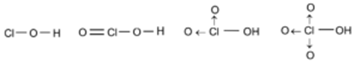
HOCl + NaOH → NaOCl + H2O
HClO4 + NaOH → NaClO4 + —– H2O
These are oxyacids of chlorine which are very strongly acidic. Chlorine has greater I.P and electronegativity. Due to the presence of 3 un-hydrogenated oxygen atoms attached to Chlorine atom, HClO4 is strongest amongst these oxyacids of chlorine.
The strengths are in the order are ClO3(OH) > ClO2 (OH) > ClO(OH) > ClOH.