In this article, we shall learn about alkanes, isomerism in alkanes. Compounds containing carbon and hydrogen only are called hydrocarbons. Examples: Methane (CH4), Ethane (C2H6), Benzene (C6H6), etc.
Aliphatic Hydrocarbons:
The hydrocarbons in which carbon atoms are joined form an open chain are called aliphatic hydrocarbons.
Examples: Methane (CH4), Ethane (C2H6), etc.
Aliphatic hydrocarbons are further classified into two types a) Saturated hydrocarbons and b) Unsaturated hydrocarbons
Saturated Hydrocarbons:
The hydrocarbons in which all valencies of each carbon atom are fully satisfied by single covalent bonds only are called saturated hydrocarbons.
Examples: Methane (CH4), Ethane (C2H6 i.e. CH3–CH3), etc.
Unsaturated Hydrocarbons:
The hydrocarbons in which valencies of at least two carbon atoms are not fully satisfied by single covalent bonds are called unsaturated hydrocarbons. They are further classified as alkenes (contain at least one carbon-carbon double bond) and alkynes (contain at least one carbon-carbon triple bond)
Alkanes:
- Open chain (aliphatic) saturated hydrocarbons are called alkanes.
- Due to their little tendency to undergo chemical changes, they are also known as paraffin.
- Their general formula is CnH2n + 2. Where n is the number of carbon atoms.
- In them, carbons are linked to each other by single covalent bonds.
- All the four valencies of carbon are satisfied by four atoms or group of atoms.
First Ten Basic Alkane:
| Sr. No. | Molecular Formula | Name of Compound | Sr. No. | Molecular Formula | Name of Compound |
| 1 | CH4 | Methane | 6 | C6H14 | Hexane |
| 2 | C2H6 | Ethane | 7 | C7H16 | Heptane |
| 3 | C3H8 | Propane | 8 | C8H18 | Octane |
| 4 | C4H10 | Butane | 9 | C9H20 | Nonane |
| 5 | C5H12 | pentane | 10 | C10H22 | Decane |
Example – 1:
Write molecular formula for alkane containing 21 carbons
Number of carbons = n = 21
Their general formula is CnH2n + 2.
Hence molecular formula of the alkane is C21H2×21 + 2. i.e. C21H44.
Example – 2:
The molecular mass of alkane was found to be 142. Find its molecular formula.
Let there are n carbon atoms in alkane, its formula is CnH2n + 2.
Molecular mass of alkane = n × 12 + (2n + 2) × 1 = 142
∴ 12n + 2n + 2 = 142
∴ 14n = 140
∴ n = 10
Hence molecular formula of the alkane is C10H2×10 + 2. i.e. C10H22.
Structural Formula:
The molecular formula of a compound indicates, the number of atoms of various elements present in the compound. But it does not give the idea about the arrangement of these atoms in space to form the molecule.
The formula which gives the exact arrangement of atoms of different elements present in the molecule of the compound is called the structural formula.
Structure of Methane Molecule:
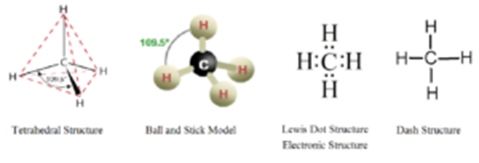
The molecular formula for methane is CH4. Thus one molecule of methane contains one atom of carbon and 4 atoms of hydrogen.
Carbon atom contains four electrons in its valence shell, while hydrogen contains one electron its valence shell. During the formation of the methane molecule, carbon atom shares its four valence electrons with four hydrogen atoms. Thus the octet of carbon and duplets of hydrogen are completed. The Structural formula for methane is as follows.
But due to SP³ hybridisation in carbon, the four valencies of carbon do not lie in the same plane. Actually, they are directed towards the four corners of a regular tetrahedron. Thus in methane, the carbon atom is at the centre of a regular tetrahedron and four hydrogens are at the four corners of this tetrahedron.
the C-C bond length is 154 pm and C-H bond length is 112 pm. the H-C-H bond is 109°28′.
Structure of Ethane:
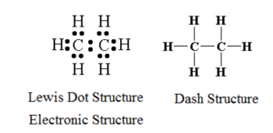
The molecular formula for methane is C2H6. Thus one molecule of ethane contains two atom of carbon and 6 atoms of hydrogen. Carbon atom contains four electrons in its valence shell, while hydrogen contains one electron its valence shell. During the formation of ethane molecule, two carbon atoms share one electron each among themselves forming carbon-carbon single bond. Rest six valence electrons ( three per carbon) are shared with six hydrogen atoms. Thus the octet of carbons and duplets of hydrogen are completed.
Classification of Alkanes:
Alkanes are classified into two types viz. Straight chain alkanes and branched chain alkanes
Straight-Chain Alkanes:
The alkanes in which all the carbon atoms are in continuous (same) chain are called straight chain alkanes or normal alkanes and are represented as n – alkanes.
CH3–CH2–CH2–CH3 CH3–CH2–CH2–CH2–CH3
n-Butane n-Pentane
Branched-Chain Alkanes:
The alkanes in which all the carbon atoms are not in continuous (same) chain are called branched-chain alkanes.
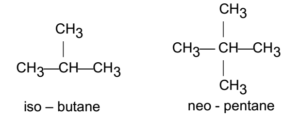
Isoalkanes:
- The alkanes in which one methyl group is attached to the second carbon atom of the normal chain of carbon atoms are called isoalkanes.
- In isoalkanes, one carbon branch is attached to the carbon atom next to the end carbon atom of the continuous chain.
- In these alkanes, CH(CH3)2– (isopropyl) group is attached at one end of the normal chain of carbon atoms.

Neoalkanes:
- The alkanes in which two methyl groups are attached to the second carbon atom of the normal chain of carbon atoms are called neoalaknes.
- In neoalkanes two carbon branches are attached to the carbon atom next to the end carbon atom of the continuous carbon chain.
- In these alkanes, H(CH3)3– (tertiary butyl group) is attached at one end of the normal chain of carbon atoms.

Types of Carbon Atoms:
Primary Carbon Atom:
Carbon atom which is attached to only one other or no carbon atom is called primary carbon. It is denoted by 1°.

Secondary Carbon Atom:
Carbon atom which is attached to two other carbon atoms is called secondary carbon. It is denoted by 2°.

Tertiary Carbon Atom:
Carbon atom which is attached to three other carbon atoms is called tertiary carbon. It is denoted by 3°.

Quaternary Carbon Atom:
Carbon atom which is attached to four other carbon atoms is called quarternary carbon. It is denoted by 4°.

Isomerism in Alkanes:
Two or more compounds having same molecular formula but different structural formulae are called isomers and the phenomenon is known as isomerism. Isomerism is a unique characteristic of organic compounds.
Alkanes show chain isomerism only. The isomerism exhibited by organic compounds due to different arrangements of carbon atoms or the nature of carbon chain in them is called chain isomerism.
Butane C4H10 has two isomers.

Pentane has three isomers

Conformations of Ethane:
The different spatial arrangements of atoms in a molecule that can be converted into one another by free rotations about a single bond are called conformations or conformers or rotamers. These isomers cannot be usually isolated because they interconvert rapidly.
By Newmann projection the carbon-carbon bond directly end on and represents two carbon atoms by circle. Bonds attached to the front carbon atom are represented by lines drawn from centre of the circle. The bonds attached to the rear carbon atom are represented by lines drawn from the edge of the circle.

These conformations are possible due to presence of carbon carbon single bond which is cylindrically symmetrical about a line joining the nuclei of the two carbons. The energy difference between these two structures is very small hence they can interconvert amomg themselves very easily.
The infinite number of intermediate conformations are called skew conformations. The energy required to rotate the molecule about carbon-carbon bond is called torsional energy. The potential energy is minimum in staggered conformation (more stable) while it is maximum at eclipse conformation (less stable). 99% ethane molecules have staggered conformation.
Each H-C-C-H unit in ethane is characterized by a torsion angle. Eclipsed bonds are characterized by a torsion angle of 0° (syn). Gauche conformation is characterized by torsion angle 60°. Anti conformation is characterized by torsion angle 180°.

Alkyl Groups:
An alkyl group is saturated hydrocarbon radical obtained by removing one hydrogen from an alkane. Alkyl group possesses one free valency which can be attached to any suitable radical. Alkyl group has a general formula is CnH2n + 1 –.
To name the alkyl group the ending characteristic –ane is replaced by –yl. Thus from methane, we get methyl group.
| Sr. No. | Alkane | Formula | Alkyl Group | Formula |
| 1 | Methane | CH4 | Methyl | CH3 – |
| 2 | Ethane | C2H6 | Ethyl | C2H5 – |
| 3 | Propane | C3H8 | Propyl | C3H7 – |
| 4 | Butane | C4H10 | Butyl | C4H9 – |
| 5 | Pentane | C5H12 | Pentyl | C5H11– |
| 6 | Hexane | C6H14 | Hexyl | C6H13 – |

IUPAC Nomenclature of Alkanes:
- The longest continuous chain of carbon atoms in the compound is selected and treated as a parent alkane and the compound is supposed to be derived from that alkane.
- If there is more than one longest chain containing the same number of carbon atoms, then the chain with maximum branching should be selected as the basic chain. Branched groups are called substituents.
- The carbons in the basic chain are then numbered such that the first substituent encountered gets the least possible number.
- The side chains (substituents) are indicated by proper prefixes to the root name.
- The location of the substituent is indicated by the number of the carbon atom to which it is attached. This number is called the locant. The locant is placed before the name of the substituent.
- The number of same substituents is denoted by proper prefix as per the table.
- The substituents are then listed alphabetically without considering the prefixes like di, tri, tetra etc.
- The comma is put to separate numbers and hyphens are put to separate a number from the letter.
- Name of last alkyl group and alkane is written as one word.