In the last article, we have studied what are alkenes and isomerism in them. In this article, we shall study different methods of preparation of alkenes.
Preparation of Alkenes By Dehydration of Alcohols:
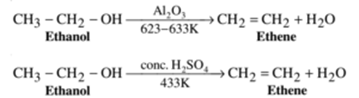
Alcohols on heating in the presence of dehydrating agents Iike concentrated sulphuric acid or phosphorous pentoxide or anhydrous alumina etc. undergoes a loss of water molecule to form an alkene. As water molecule is removed from the alcohol molecule, the reaction is called a dehydration reaction. The ease of dehydration in alcohol is in the following order:
Tertiary alcohol > Secondary alcohol > Primary alcohol
General Reaction:

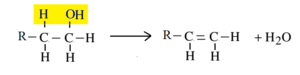
Example – 1: Preparation of ethene from ethyl alcohol (Ethanol):
Example – 2: Preparation of propene from n-propyl alcohol (Propan-1-ol):

Example – 3: Preparation of propene from iso-propyl alcohol (Propan-2-ol):

Example – 4: Preparation of isobutylene (2-Methylprop-1- ene) from tert – butyl alcohol (Propan-2-ol):

Reaction Mechanism:
Acid is proton donor. A proton (H+) from acid acts on the most electronegative atom in alcohol. i.e. oxygen and gets attached to it. This process is called protonation of alcohol.

A water molecule is eliminated by protonated alcohol to give a positively charged species called carbocation.

The carbocation formed is unstable and loses a proton to form an alkene.

Preparation of Alkenes By Dehydro-Halogenation of Alkyl Halides:
Alkyl halide on refluxing with alcoholic KOH, undergoes a loss of hydrogen halide, to form an alkene. The ease of dehydro-halogenation in alcohol is in the following order.
For same alkyl group and different halagen
R-I > R-Br > R-Cl
For different alkyl group but same halogen
Tertiary alkyl halide > Secondary alkyl halide > Primary alkyl halide
General Reaction:

Example – 1: Preparation of Ethene From Ethyl Bromide (Bromoethane):

Example – 2: Preparation of propene From n-Propyl chloride (1-Chloropropane):

Example – 3: Preparation of propene From iso-Propyl Iodide (2-Iodopropane):

Example – 4: Preparation of 2-Methylpropene From tert-Butyl bromide:

Saytzeff’s Rule:
In dehydrohalogenation or in dehydration, an alkene formed by elimination has a greater number of alkyl groups attached to the doubly bonded carbon atom.

Preparation of Alkenes By Cracking Alkanes:
The higher alkane when heated strongly in the absence of air decomposes to give a lower alkene and lower alkane or hydrogen. Temperature is maintained at 500 °C and catalyst used is silica-alumina.
Example -1: Ethane when heated strongly in the absence of air, decomposes to give ethene.

Example-2: When Propane when heated in the absence of air, using silica- aluminium as catalyst decomposes to give a mixture of propene and methane.

Preparation of Alkenes By Dehalogenation of Vicinal Dihalides:
Dihalogen derivatives of alkanes in which the two halogen atoms are attached to two neighbouring carbon atoms are called vicinal dihalides.
When vicinal dihalides are heated with zinc dust in the presence of ethanol, alkenes are obtained.
General Reaction:

Example – 1: Preparation of Ethene:

Example – 2: Preparation of Propene:

Preparation of Alkenes By Kolbe’s Electrolysis:
Electrolysis of aqueous solutions of sodium or potassium salts of saturated dicarboxylic acids gives an alkene. When an aqueous solution of sodium or potassium salt of a dibasic acid is electrolyzed, an alkene is produced. For example, electrolysis of sodium succinate gives ethene.

Preparation of Alkenes By Controlled Hydrogenation of Alkynes:
Use of Catalyst and Heat:

Use of Lindlar’s Catalyst:
Lindlar’s catalyst is palladium (Pd) supported over calcium carbonate or charcoal and partially deactivated with poisons such as sulphur or quinoline. When Lindlar’s catalyst is used major product is cis alkene

Use of sodium and liquid ammonia:
When sodium and liquid ammonia is used the major product is a trans alkene

Physical Properties of Alkanes:
Physical State: First three members of alkenes viz. Ethene, Propene, and Butene are colourless gases. Alkenes containing 5 to 17 carbons are liquids and higher members are solid at room temperature.
Odour (Smell): Alkenes are odourless except Ethene which has a pleasant smell. Higher alkenes are odourless and colourless.
Solubility: Alkenes are insoluble in water but are readily soluble in non-polar organic solvents like benzene, hexane, CCl4, etc.
Combustivity: Alkenes burn in air with luminous flame, producing carbon dioxide and water.
Melting and Boiling Points: Alkenes have low melting and boiling points. The melting point and boiling point rises with increases in the molecular weight. For every -CH2 added the boiling point increases by 20- 30 K. Cis isomers have a higher boiling point than corresponding trans isomer. Generally melting point of cis isomer is lower than the corresponding trans isomer.

Density: There is a gradual increase in densities with the increase in the molecular mass. Alkenes are lighter than water and their maximum density is 0.8. The alkene gases and vapours form an explosive mixture with air.