Science > Biology > Human Anatomy and Physiology > Cardiovascular System > Composition of Blood: Erythrocytes
When a human blood sample is prevented from clotting and spun in a test tube (centrifuged), in a machine called a centrifuge, the blood separates into a straw coloured liquid called plasma and a dark brown mass of blood cells. The lower layer consists of white blood cells, blood platelets, and red blood cells. Collectively, these are the formed elements, which make up about 45% of the total volume of whole blood. About 95% of the volume of the formed elements consists of red blood cells or erythrocytes. The remaining 5% consists of white blood cells or leukocytes, and cell fragments called platelets or thrombocytes. The percentage of blood attributed to red blood cells is called the hematocrit. The hematocrit is defined as the percentage of blood volume that is occupied by erythrocytes. The normal hematocrit is approximately 45 percent in men and 42 percent in women.
The process of blood cell production called hematopoiesis or hemopoiesis. It occurs in the embryo and fetus in tissues like the yolk sac, liver, thymus, spleen, lymph nodes, and red bone marrow. After birth, hematopoiesis is confined primarily to red bone marrow, with some lymphoid tissue helping in the production of lymphocytes. In young children, nearly all the marrow is red bone marrow. In adults, however, red marrow is confined to the ribs, sternum, vertebrae, pelvis, proximal femur, and proximal humerus. Yellow marrow replaces red marrow in other locations in the body.
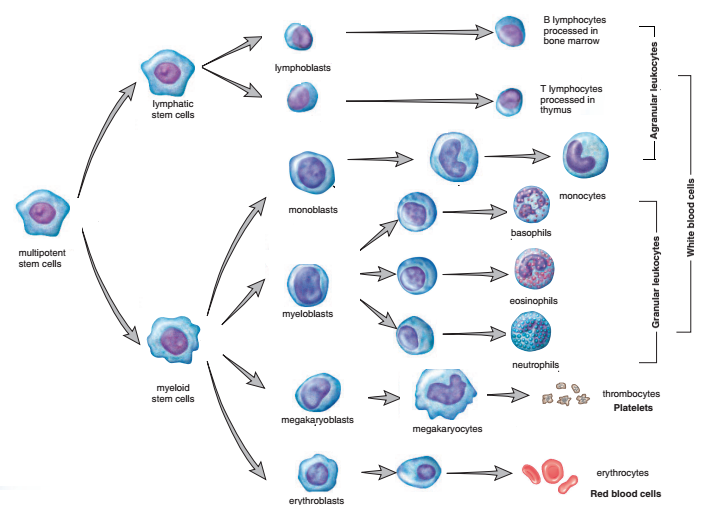
All the formed elements of the blood are derived from a single population of stem cells located in the red bone marrow. Hemopoietic stem cells are precursor cells capable of dividing to produce daughter cells that can mature differentiate into various types of blood cells: proerythroblasts, from which red blood cells develop; myeloblasts, from which basophils, eosinophils, and neutrophils develop; lymphoblasts, from which lymphocytes develop; monoblast, from which monocytes develop; and megakaryoblasts, from which platelets develop. A multipotent stem cell that divides, producing two other types of stem cells. The myeloid stem cell gives rise to the cells that go through a number of stages to become red blood cells, platelets, granular leukocytes, and monocytes. The lymphatic stem cell produces the lymphocytes.
Red Blood Corpuscles:
Red blood cells or erythrocytes or RBCs are small, biconcave disks that are, a disk thicker at the edges than in the middle, like a doughnut with a center depression on each side instead of a hole. This shape and their small size (7 micrometers in diameter) impart to the erythrocytes a high surface-to-volume ratio so that oxygen and carbon dioxide can diffuse rapidly to and from the interior of the cell. The plasma membrane of erythrocytes contains specific polysaccharides and proteins that differ from person to person, and these lead to the formation of different blood groups. They lack a nucleus when mature. In addition, the red blood cell can bend or fold around its thin center, thereby decreasing its size and enabling it to pass more easily through small blood vessels.

The number of erythrocytes is counted by the instrument called hemocytometer. Erythrocytes are about 700 times more numerous than white blood cells and 17 times more numerous than platelets in the blood. Males have about 5.4 million red blood cells per mm3 of blood (range: 4.6–6.2 million), whereas females have about 4.8 million per mm3 (range: 4.2–5.4 million). For the persons living at high altitudes and persons engaged in hard physical work, the RBC count is high.
Red blood cells transport oxygen, and each contains about 200 million molecules of hemoglobin, the respiratory pigment. RBCs do not contain a nucleus, mitochondria, endoplasmic reticulum, and Golgi complex. Hence more space is available for oxygen-carrying pigment haemoglobin (hemoglobin). Red blood cells cannot move of their own accord and are passively moved by forces that cause the blood to circulate.
They give a red colour to the blood. The red colour is due to pigment haemoglobin. Haemoglobin maintains the pH of the blood and acts as a buffer. Less amount of haemoglobin results in anaemia. Individual RBC appears yellowish in colour but in bulk, they give a red colour to the blood.
Structural Advantages of RBCs:
- Biconcave Nature: The biconcave nature of red blood cells is due to the loss of the nucleus. It increases their surface area and assists in better oxygen diffusion.
- Absence of Nucleus: Due to the absence of nucleus RBCs remain flexible and can squeeze through small blood capillaries.
- Loss of Mitochondria: The lack of nucleus and mitochondria means that red blood cells must get their energy differently using a process called glycolysis to produce ATP (anaerobic respiration) followed by lactic acid production. Due to the absence of mitochondria, the oxygen absorbed by the haemoglobin is not used in the cell for producing energy and almost all the oxygen absorbed, can be delivered to the cell. Due to the absence of mitochondria RBCs remain flexible and can squeeze through small blood capillaries.
- Small, Elastic Disc-like Structure: The diameter of the capillary is smaller than the red blood cell. Due to the special shape, the cell can pass through the capillary after getting folded, squeezed, twisted,
Structural Disadvantages of RBCs:
Due to the lack of nuclei and organelles, the mature red blood cells do not contain DNA and cannot synthesize any RNA, and consequently, they cannot divide or repair themselves, limiting their lifespan.
Life Cycle of RBCs:
Formation of RBCs:
The formation of RBCs is called erythropoiesis. RBCs are formed in the soft interior of bones called bone marrow, specifically the “red” bone marrow under the influence of hormone erythropoietin (formed in the kidneys). When mature they lose nucleus and other organelles. Naturally, only mature erythrocytes, which have lost ribosomes, leave the bone marrow and enter the general circulation. Erythropoietin is normally secreted in relatively small amounts, which stimulate the bone marrow to produce erythrocytes at a rate adequate to replace the usual loss. The erythropoietin secretion rate is increased markedly above basal values when there is a decreased oxygen delivery to the kidneys. At high altitude oxygen is less. At a high altitude, there is red blood cell loss, or has impaired lung function. To overcome this the kidneys accelerate their release of erythropoietin, which stimulates stem cells and speeds up the maturation of red blood cells.

The production of erythrocytes requires the usual nutrients needed to synthesize any cell: amino acids, lipids, and carbohydrates. In addition, both iron and certain growth factors, including the vitamins folic acid and vitamin B12, are essential. Production of normal erythrocyte numbers requires an extremely small amount of a cobalt-containing molecule, vitamin B12 (also called cobalamin). It is required for the action of folic acid.
Life Span and Breakdown:
Because erythrocytes lack nuclei and organelles, they can neither reproduce themselves nor maintain their normal structure for very long. The average life span of an erythrocyte is approximately 120 days, which means that almost 1 percent of the body’s erythrocytes are destroyed and must be replaced every day. The breakdown of old and worn out RBCs takes place mainly spleen and liver where they are engulfed by macrophages. The process of the breakdown of old and worn out RBCs is called haemolysis.
The globin portion of the hemoglobin is broken down into its component amino acids, which are recycled by the body. The iron is recovered and returned to the bone marrow for reuse. The breakdown of the protein part of RBCs forms biliverdin and then converts into bilirubin, which is released into the plasma. The straw colour of the plasma is due to the presence of the pigment bilirubin. Bilirubin binds to albumin and is transported to liver cells. This bilirubin is called free bilirubin because it is not yet conjugated. Free bilirubin is taken up by the liver cells and is conjugated, or joined, to glucuronic acid to form conjugated bilirubin, which is more water-soluble than free bilirubin. The conjugated bilirubin becomes part of the bile, which is the fluid secreted from the liver into the small intestine. In the intestine, bacteria convert bilirubin into the pigments that give feces its characteristic brownish color.

Functions of RBCs:
- The primary functions of red blood cells are to transport oxygen from the lungs to the various tissues of the body and to transport carbon dioxide from the tissues to the lungs. Approximately 98.5% of the oxygen transported in the blood is transported in combination with the hemoglobin in the red blood cells, and the remaining 1.5% is dissolved in the water part of the plasma.
- It carries carbon dioxide from the tissues to the lungs. 23% of carbon dioxide formed in tissues is carried to the lungs by this method.
- They play an important role in the clotting of blood.
- They carry cells and antibodies to the site of action that fights infection.
- Haemoglobin is an excellent acid-base buffer and hence maintains the pH of the blood.
If red blood cells rupture, the hemoglobin leaks out into the plasma and becomes nonfunctional because the shape of the molecule changes as a result of denaturation. Red blood cell rupture followed by hemoglobin release is called hemolysis.
Haemoglobin (Hemoglobin):
It is iron-containing conjugated protein and oxygen-carrying pigment in RBCs. Its empirical formula is C2952H4664N812O832S8Fe4. It is a macromolecule and has a molecular mass greater than 66000.
Hemoglobin consists of four polypeptide chains and four heme groups. Each polypeptide chain, called a globin, is bound to one heme. Each heme is a red-pigment molecule containing one iron atom. Heme is an iron porphyrin compound. Porphyrin is a tetrapyrrole structure (Pyrroles are a five-atom ring with four carbon and one nitrogen atoms). Ferrous iron occupies the centre of the porphyrin ring and establishes linkages with all the four nitrogens of all the pyrrole rings. It is also linked to the nitrogen of the imidazole ring of histidine present in the globin part. Several types of globin exist, each having a slightly different amino acid composition. The four globins in normal adult hemoglobin consist of two alpha ( α ) chains and two beta ( β ) chains.

Iron:
Iron is very important in the production of haemoglobin awhich is an oxygen carrier. Small amounts of iron are lost from the body via the urine, feces, sweat, and cells sloughed from the skin. In addition, women lose an additional amount of iron via menstrual blood. This iron lost from the body must be replaced by the ingestion of iron-containing foods like meat, liver, shellfish, egg yolk, beans, nuts, and cereals. The body has a considerable store of iron, mainly in the liver, bound up in a protein called ferritin. Ferritin serves as a buffer against iron deficiency. A significant upset of iron balance can result either in iron deficiency, leading to inadequate hemoglobin production, or in an excess of iron in the body, with serious toxic effects (hemochromatosis).
The haemoglobin count in blood is measured by an instrument known as haemometer or haemoglobinometer. In human adult males has haemoglobin count of 15.0 g per 100 mL, for females 10.0 to 14.0 g per 100 m and for infants 16.5 – 19.5 g per 100 mL.
When hemoglobin is exposed to oxygen, one oxygen molecule can become associated with each heme group. This oxygenated form of hemoglobin is called oxyhemoglobin. Oxyhemoglobin is bright red. Hemoglobin containing no oxygen is called deoxyhemoglobin. Deoxyhemoglobin has a darker red color.
Haemoglobin forms an unstable, reversible bond with oxygen. In its oxygenated state, it is called oxyhemoglobin and is bright red.
Haemoglobin + Oxygen ⇌ Oxyhaemoglobin
RBCs carrying oxygen travel to tissues in different parts of the body. In the tissues oxyhaemoglobin readily liberates oxygen.
Oxyhaemoglobin ⇌ Haemoglobin + Oxygen
A small amount of carbon dioxide is also transported by haemoglobin to the lungs, which doesn’t combine with the iron atoms but is attached to amino groups of the globin molecule. This hemoglobin form is carbaminohemoglobin
A recently discovered function of hemoglobin is the transport of nitric oxide, which is produced by the endothelial cells lining blood vessels.
Previous Topic: Composition of Blood: Blood Plasma
Next Topic: Disorders Related to RBCs (Anemia)