Science > Physics > Photoelectric Effect > Photoelectric Effect
It is found that when the light of very short wavelength (or high frequency) is incident on a certain metallic surface of photosensitive material, electrons are emitted by the surface. Most of the metals emit electrons when ultra-violet light is incident on them. However, alkali metals like sodium, potassium, etc. emit electrons even when ordinary light falls on them. These materials are known as photosensitive materials. In this article, we shall study, the photoelectric effect.
This phenomenon of emission of electrons by a certain metal surface when radiation of suitable frequency is incident on it is called photoelectric effect. The electrons emitted from the metal surface are called photo-electrons. And the current is called photoelectric current or photocurrent. This phenomenon was first discovered by Hertz in 1887.
Experimental Study of Photoelectric Effect:
Circuit Arrangement:
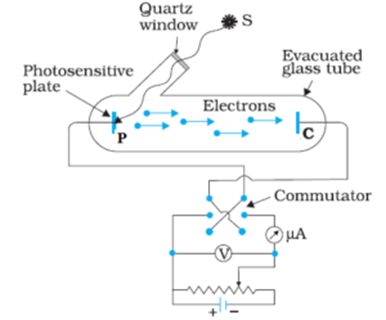
The experimental arrangement to study the photoelectric effect is as shown in the diagram. The apparatus consists of an evacuated glass tube. Two metal electrodes plate P and collector C, are enclosed in this tube. This glass tube is provided with a side quartz window. The plate A is connected to the positive terminal of a battery and the cathode is connected to the negative terminal of the battery. An ultra-violet light of variable frequency is made incident on the plate through the window. The plate acts as a photoelectron emitter. When the ultra-violet light of suitable frequency is incident on the plate, it emits photoelectrons which starts flowing through the external circuit and constitute the photoelectric current.
Study of the Effect of the Frequency of the Incident Light on Photoelectric Effect:
A suitable potential difference is applied between plate P and collector C. Light of constant intensity but the variable frequency is allowed to fall on plate P. The frequency of the incident light is slowly increased. It is found that there would be no photoelectric current up to certain limiting frequency. This frequency is called threshold frequency ( ν 0). The value of the threshold frequency depends on the material of the plate P.
The minimum frequency of incident radiation for which photoelectrons are just emitted from the photosensitive material is called threshold frequency. The variation of photoelectric current with the frequency of incident radiation is as shown in the following graph.
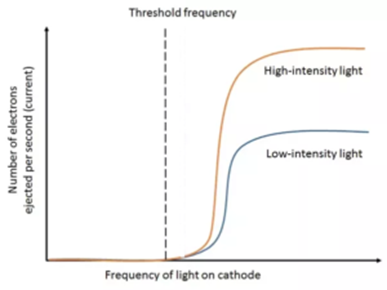
This shows that the photo-electric effect depends upon the frequency of the incident light (radiation). The threshold frequency is different for different materials of the emitter and it is a characteristic property of that material.
Study of the Effect of Intensity of Incident Light on Photoelectric Effect:
A suitable potential difference is applied between plate P and collector C. Light of constant frequency having frequency more than the threshold frequency of material of emitter P but of the variable intensity is allowed to fall on plate P. The intensity of the incident light is slowly increased. As the intensity of incident light increases the photoelectric current increases. This shows that the photo-electric effect depends upon the intensity of the incident light (radiation).
The variation of photoelectric current with the intensity of incident radiation is as shown in the following graph.

Study of the Effect of Potential Difference Between the Emitter and Collector on Photoelectric Effect:
Light of constant frequency having frequency more than the threshold frequency of material of emitter P and constant intensity is allowed to fall on plate P. A variable potential difference is applied between plate P and collector C. The positive potential difference applied to the emitter P and collector C initially slowly increased zero, the photoelectric current increases. At a certain positive potential of plate A, all electrons emitted are collected by plate A and the photoelectric current becomes maximum. If we increase the positive potential of the plate, the photoelectric current remains constant. This maximum value of photoelectric current is called saturation current.
The variation of photoelectric current with the potential difference between the plate and collector is shown in the following graphs.

If the positive potential difference applied to the emitter P and collector C is slowly decreased to zero. As the potential difference applied between plate P and collector C decreases the photoelectric current decreases.
This shows that all photo-electrons are not emitted with the same kinetic energy and the maximum kinetic energy of the photoelectrons depends upon the frequency of incident light.
If the potential of the plate is reduced below zero and it is made more and more negative, a point will be reached when the photoelectric current reduces to zero. The magnitude of this retarding potential for which photoelectric current is zero is called stopping potential Vo. The value of stopping potential depends on the kinetic energy of photoelectrons.
We have,

Threshold Frequency and Threshold Wavelength:
It is found that there would be no photoelectric current up to certain limiting frequency. This frequency is called threshold frequency (νo). The value of the threshold frequency depends on the material of the plate P.
The minimum frequency of incident radiation for which photoelectrons are just emitted from a photosensitive material is called threshold frequency. The corresponding wavelength of the radiation is called the threshold wavelength. The maximum wavelength of incident radiation for which photoelectrons are just emitted from the photosensitive material is called threshold wavelength (λo).
Concept of Saturation Current:
The positive potential difference applied to the emitter P and collector C slowly increased zero, the photoelectric current increases. At a certain positive potential of plate A, all electrons emitted are collected by plate A and the photoelectric current becomes maximum. If we increase the positive potential of the plate, the photoelectric current remains constant. This maximum value of photoelectric current is called saturation current. For constant frequency of radiation, as the plate potential increases the saturation current increases.
Concept of Stopping Potential:
If the potential of the plate is reduced below zero and it is made more and more negative, a point will be reached when the photoelectric current reduces to zero. The magnitude of this retarding potential for which photoelectric current is zero is called stopping potential Vo. The value of stopping potential depends on the kinetic energy of photoelectrons. We have,

Where, e = Charge on electron
Vo = Stopping potential
m = Mass of electron
vmax= Maximum velocity of photoelectron
For the constant potential of the plate, as the plate frequency of incident radiation increases the stopping potential increases.
Variation of Stopping potential with Frequency:
The following graph shows the variation of stopping potential with the frequency of incident radiations for two metals say A and B.

Conclusions:
Stopping potential at threshold frequency is zero and the stopping potential varies directly with the frequency of incident radiation.
Characteristics of the Photoelectric Effect:
- For a given metallic surface, photo-electrons are emitted only when the frequency of incident light is greater than or equal to a certain minimum frequency (νo) known as the threshold frequency. The threshold frequency is different for different Substances,
- If the frequency of incident light is less than the threshold frequency, photoelectrons are not emitted, however large the intensity of incident light may be.
- The number of photo-electrons emitted per second is directly proportional to the intensity of incident light. Thus photoelectric current is directly proportional to the intensity of incident light.
- Photo-electrons are emitted with different velocities. The maximum velocity (and hence maximum K.E.) of a photo-electron depends upon the frequency of incident light and does not depend upon its intensity.
- The maximum K.E. of the photo-electron increases with an increase in the frequency of incident light.
- The photoelectric effect is an instantaneous process. There is no time lag between the incidence of light and the emission of the photo-electrons in other words, the surface begins to emit photo-electrons as soon as light falls on it. Also, the emission of photo-electrons stops the moment incident light is cut off.
- In the case of alkali metals like sodium, potassium, etc. first ionization potential (ionization enthalpy) is low and hence emit electrons even when ordinary light falls on them. These materials are known as photosensitive materials.
Note:
Frequencies of radio waves are low, they are lower than threshold frequencies of alkali metals (having lowest threshold frequencies). Hence no photoemission is possible with radio waves.
Previous Topic: Numerical Problems on Specific Charge (e/m ratio)
Next Topic: Numerical Problems on Photoelectric Effect
2 replies on “Photoelectric Effect”
this site is very useful for me!!
Very useful…