The group IV-A (14) in the periodic table is known as ‘Carbon family’ consist of five elements, namely, Carbon (C), Silicon(Si) Germanium (Ge), Tin (Sn) and Lead (Pb).
Carbon is the essential constituent of all organic matter, while silicon is an important constituent of inorganic matter.
Electronic Configuration of Group IV(14) Elements:
| Sr.No. | Elements | Atomic No. | Electronic Configuration | Configuration of valence shell |
| 1 | Carbon (C) | 6 | 2, 4 | 2s2 2p2 |
| 2 | Silicon (Si) | 14 | 2, 8, 4 | 3s2 3p2 |
| 3 | Germanium (Ge) | 32 | 3, 8,18, 4 | 4s2 4p2 |
| 4 | Tin (Sn) | 50 | 2, 8, 18, 8, 4 | 5s2 5p2 |
| 5 | Lead (Pb) | 82 | 2, 8, 18, 32, 18, 4 | 6s2 6p2 |
Notes:
- The similarities in the properties of these elements &rise due to the similarity in their outer configuration ns2 np2.
- The differences and gradation in properties arise due to their atomic radii and the number of electrons in the penultimate shell. Carbon has 2 electrons. Silicon has 8 electrons and other elements have 18 electrons In their penultimate shell.
- This causes a difference in the physical properties of Carbon and Silicon on one hand and Ge, Sn, Pb on the other hand.
- Silicon differs from Carbon having vacant 3d orbitals. Hence it is able to show covalency greater than four
Position of Silicon in the Periodic Table:
- Atomic Number of silicon is 14. Detailed Electronic Configuration: 1s2, 2s2 2p6, 3S2, 3px1 , 3 py1, 3 pz0
- The last electron enters into ‘p’ sub-shell, therefore, Silicon belongs to ‘p’ block normal non-metallic element.
- The total number of orbits = 3. Therefore, silicon belongs to 3rd period.
- It has four electrons in the valence shell. Therefore, it is placed in the Group IV-A (14) of the periodic table. This is transition position between metals and non-metals.
Electronic Configuration of Silicon:
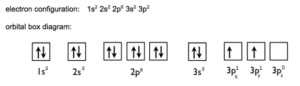
Ground State:
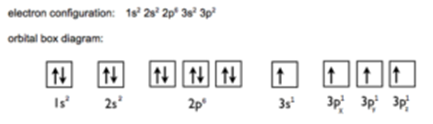
Excited state:
Hybridized State:

Oxidation States of Silicon:
- Electronic configuration of ‘Si’ in ground state is 1s2, 2s2 2p6, 3S2 3px1 3 py1
- Electronic configuration of ‘Si’ in excited state is 1s2, 2s2 2p6, 3S2 3px1 3 py1 3 pz1
- Silicon undergoes sp3 hybridization and forms four half-filled sp3 hybrid orbitals which are used to form four covalent bonds.
- Because of the high Ionization potential existence of (Si4+) ion is unlikely. Silicon shows +4 oxidation states by sharing four outer electrons with strongly electronegative elements like halogens and oxygen.

Silicon exhibits -4 oxidation state in silicides. Silicides are formed at high temperature

Silicon has vacant 3d orbitals. Hence it is possible for silicon to show a covalency greater than four.
Oxidation States of Group IV-A (14) Elements:
Since they possess high ionization energies, the existence of (4+) ions is unlikely. Their electronegativity values are also low. Therefore (4-) ions also do not form normally. Only carbon and Silicon exhibit (4-) oxidation state. They show (4+) oxidation state by forming covalent bonds with strongly electronegative elements. For this purpose sp3 hybrid orbitals are used.
They also show (2+) oxidation state by sharing their two unpaired np2 electrons, (ground state). From carbon to lead, with the increase in nuclear ‘charge, stability of ns2 electron pair increases. Hence the stability of lower oxidation state (2+) increases and that of higher oxidation state (4+) decreases from carbon to lead. This is due to inert pair effect.
Metallic and Non-metallic Character of Group IV-A (14) Elements:
The first two elements carbon and silicon are distinctly non-metallic. Germanium is a metalloid. Tin and Lead are well-defined metals. Silicon has most of the characteristics of non-metals. But it is a semiconductor at room temperature, therefore- sometimes it is considered as a metalloid. Metallic nature or electropositive nature increases from carbon to lead.
Electronegativity and Ionisation Potential (Ionization Enthalpy) of Group IV-A (14) Elements:
With the increase in atomic number i.e. from carbon to lead, as the number of shells increases, their atomic radii also increases. Ionization potential and electronegativity decrease with an increase in their atomic radii.
Hardness:
Because of small atomic radii, in carbon and silicon there are strong inter-atomic forces of attraction and hence they are hard solids with high melting and boiling points. Germanium is quite hard, but tin and lead are soft silvery-white metals.
Allotropy:
All the elements except Ge and Pb show allotropy
Catenation:
Carbon can combine with other carbon atoms to form quite large carbon chains. Silicon has a smaller capacity to form chains. Germanium has still a smaller tendency while tin and lead have hardly any tendency to do so.
Silicon
It derives its name from the Latin word Silex meaning hard stone. Next to oxygen, it is the most abundant element in the earth’s crust. It forms 26% of the mass of the earth’s crust and is widely spread in nature.
Occurrence:
Silicon does not occur in nature in the free state. It occurs in nature as Silica (Si02) in the form of sand, quartz, flint, agate, and opal. As Natural complex silicates
- Feldspar : K2O . A2O3 . 6 SiO2
- Mica: 3K20 . 3Al2O3 . 6 SiO2 . 2 H2O
- Kaolin or China Clay : Al2O3 . 2 SiO2 . 2 H20
- Asbestos: CaO. 3MgO. 4SiO2
Physical Properties of Silicon:
- The physical properties of silicon are as follows.
- It exists in two allotropic forms – amorphous and crystalline.
- The amorphous form is common. It is a brown powder with a specific gravity of 2.35.
- The crystalline form is steel grey coloured with specific gravity 2.50.
- Though inactive, amorphous silicon is more reactive than crystalline form.
- Its melting point is 1693 K (1420°C) and its boiling point is 2873 K (2600°C).
- Both the forms are poor conductors of electricity but conductivity increases with temperature. (it is a semiconductor).
- Its crystals are hard enough to scratch glass.
- It is insoluble in water and in any single acid. But a mixture of hydrofluoric acid and nitric acid reacts with it. It reacts with fused alkalies or hot boiling solutions of alkalies.
Preparation of Silicon:
Preparation of Amorphous Silicon from Silica:
Finely powdered pure silica or quartz is mixed with a requisite quantity of magnesium powder. The mixture is then heated in a fire clay crucible in the absence of air. The reduction of silica takes place as
SiO2 + 2Mg → 2MgO + Si
After cooling, the product is treated with dil. HCI which dissolves MgO and unreacted Mg.
MgO + 2HCI → MgCl2 + H2O
Mg + 2HCI → MgCl2 + H2O
The insoluble mass is then treated with hydrofluoric acid. The unchanged SiO2, is converted into volatile SiF4.
SiO2 + 4HF → SiF4 + 2 H20
Si remains unaffected by HCl and HF. It is then washed with water and dried in a current of H2 gas. The process gives silicon as a brown amorphous powder.
Preparation of Amorphous Silicon from Silicon Tetrachloride:
Pure sample of amorphous silicon can be prepared by passing vapours of silicon tetrachloride over molten sodium metal in an inert atmosphere.
SiCl4 + 4Na → Si + 4NaCl
The product is washed with water to remove NaCl and unreacted sodium. The product is dried in a current of H2 gas. Silicon is left behind as brown amorphous powder.
Preparation of Crystalline Silicon from Potassium Silicofluoride:
A mixture of potassium fluosilicate or potassium silicofluoride and excess of aluminium powder is heated at 1273 K.
3K2SiF6 + 4Al → 3Si + 6KF + 4AIF3
AlF3 volatilises off. Silicon formed dissolves in molten aluminium. As the molten mass cools, Si crystallises out. The mass is then heated with dil. HCI to remove unreacted aluminium.
2Al + 6HCl → 2AICl3 + 3H2O
It is then washed with water. (KF is water soluble). The process gives lustrous grey crystalline Si.
Reactions of Silicon:
Reaction with alkalies:
it dissolves in the aqueous hot alkali solution to form alkali silicate and hydrogen.
Si + 2 NaOH + H2O → Na2SiO3 (Sodiumsilicate) + 2H2 ↑
Reaction with Sodium Carbonate or Washing Soda:
When fused with Sodium carbonate, it forms sodium silicate by displacing carbon and elementary carbon is set free.
Na2CO3 + Si → Na2SiO3 (Sodiumsilicate) + C
Reaction with Metals:
At the temperature of the electric furnace it directly combines with Magnesium, Copper and Chromium forming Silicides.

Reaction with Halogens:
it burns spontaneously in fluorine at room temperature forming Silicon tetrafluoride.
Si + 2F2 → SiF4 (Silicontetrafluoride)
Si + 2Cl2 → SiCl4 (Silicontetrachloride)
Action of Steam:
When steam is passed over red-hot silicon, silicon dioxide is obtained with evolution of hydrogen gas
Si + 2H2O → SiO2 + 2H2 ↑
Uses of Silicon:
- It is used as deoxidiser in metallurgy in making castings of steel, copper and bronze.
- Its major use is in the semiconductors which are the main components of transistors. Transistors are employed in a variety of electronic devices such as Radio, T.V. sets, Computers, Solar batteries.
- It is used in the preparation of important alloys.
- Ferro-silicon: it is used as a deoxidizer in the manufacture of steel.
- Silicon-steel: Steel containing 5% Si is soft and magnetic. It is used for making magnetic cores.
- Silicon-bronze: It is used in the manufacture of telegraphic and telephone wires.
- It is used in the preparation of refractory materials like Crucibles.
Simple Silicates:
Silicates are compounds containing silicon, oxygen and metal or metals. They contain Si-O-Si linkage or Si-O bond in their structure.
Simple silicates can be considered as the metal salts of orthosilicic acid H2SiO4. Simple silicates are orthosilicates consisting of metal cations such as Na+ , Mg2+, Zn2+ etc. and silicate anion (SiO4)4-. Example: Mg2(SiO4). All silicates contain tetrahedral (SiO4)4- units.
The silicates having only discrete (SiO4)4- units are considered as simple silicates. The silicates containing two or more (SiO4)4- units linked through oxygen atom are generally considered as complex silicates. e.g. pyrosilicates having (Si2O7)6- anion.
(SiO4)4- Unit Present in Silicates have Tetrahedral Geometry:
Electronic, configuration of silicon atom in its ground state is 1s2, 2s2 2p6, 3S2, 3px1 , 3 py1, 3 pz0. At the time of combination, one of the 3s electron gets promoted to the empty 3p. This excited state of silicon has electronic configuration 1s2, 2s2 2p6, 3S1, 3px1 , 3 py1, 3 pz1
Si undergoes sp3 hybridisation and forms four sp3 hybrid orbitals which are directed to the corners of regular tetrahedron at the centre of which there is a Si atom.
The electronic configuration of oxygen atom is 1s2, 2s2 , 2px2 , 2 py1, 2 pz1 . Oxygen has two unpaired electrons in two of its ‘p’ orbitals (2py, 2pz)
Each sp3 hybrid orbital of silicon atom overlaps with one of the 2p orbital (2py, 2pz) of the oxygen atom that has the unpaired electron and forms a covalent Si-O bond. Si-O covalent bond is sp3-p- sigma bond. Si forms such four bonds in (SiO4)4- units.
Thus silicon completes its octet by forming four covalent bonds with four oxygen atoms. Each oxygen atom is still short of one electron to complete the octet. In order to complete the octet, the oxygen atoms pick up one electron each from some metal. In this process, oxygen atom becomes -1.This gives rise to the anion (SiO4)4-.
Since the formation of (SiO4)4- unit involves sp3 hybridisation of Si, it has tetrahedral structure. Silicon atom lies at the centre and four oxygen atoms lie at the four corners of the regular tetrahedron.
Diagram :

Strength of Si-O Bond in Simple Silicates:
Si and O bond in silicates is covalent. The electronegativity difference between silicon (EN = 1.8) and Oxygen (EN = 3.5) is 1.7 pauling. Due to this Si-O bond becomes polar. So it gets an ionic character (about 30%) Because of this ionic character the bond has a greater strength and stability and more energy is required to break the bond.
Only HF can break Si-O bond. Fluorine (EN = 4) is more electronegative than oxygen (EN = 3.5) and H-F is more ionic than Si-O bond. As a result, Si-O is broken by H-F only to form a more stable Si-F bond.
One reply on “Silicon”
well explained. thankks