In this article, we shall learn about alkanes, isomerism in alkenes. Compounds containing carbon and hydrogen only are called hydrocarbons. Examples: Methane (CH4), Ethane (C2H6), Benzene (C6H6), etc.
Aliphatic Hydrocarbons:
The hydrocarbons in which carbon atoms are joined form an open chain are called aliphatic hydrocarbons.
Examples: Methane (CH4), Ethane (C2H6), etc.
Aliphatic hydrocarbons are further classified into two types a) Saturated hydrocarbons and b) Unsaturated hydrocarbons
Saturated Hydrocarbons:
The hydrocarbons in which all valencies of each carbon atom are fully satisfied by single covalent bonds only are called saturated hydrocarbons. Examples: Methane (CH4), Ethane (C2H6 i.e. CH3–CH3), etc.
Unsaturated Hydrocarbons:
The hydrocarbons in which valencies of at least two carbon atoms are not fully satisfied by single covalent bonds are called unsaturated hydrocarbons. They are further classified as alkenes (contain at least one carbon-carbon double bond) and alkynes (contain at least one carbon-carbon triple bond)
Alkenes:
- Alkenes are unsaturated aliphatic hydrocarbons having one or more carbon-carbon double bonds i.e. C=C. Since alkenes form oily products with halogens, they are called olefins.
- They contain two hydrogen atoms less than the corresponding alkanes. The general formula for alkenes is CnH2n, Where n = number of carbon atoms.
- The aliphatic hydrocarbons containing two or three carbon-carbon double bonds are called alkadienes and alkatrienes respectively.
- Example of alkenes:
- CH2CH2 Ethene or Ethylene
- CH3 – CH=CH2 Propene or Propylene
Isomerism in Alkenes:
Structural Isomerism in Alkenes:
Chain Isomerism:
Chain isomerism is due to the difference in the structure of the carbon chain in alkenes.
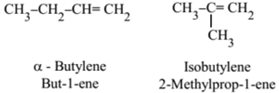
Position Isomerism:
It is due to the difference in the position of the double bond in the same carbon chain
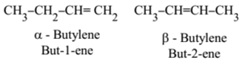
Geometric Isomerism:
The isomerism which is due to restricted rotation about carbon-carbon double bond is known as geometrical isomerism or cis-trans isomerism.
In alkenes, it is seen that free rotation about the carbon-carbon single bond is possible. However, in alkenes, such a rotation is not possible because any rotation about the axis for carbon-carbon (sigma) bond would decrease the overlapping of orbitals and a large amount of energy necessary to do this is not available under ordinary conditions. This gives rise to restricted rotation about the carbon-carbon double bond. This restricted rotation about the carbon-carbon double bond causes the two different spatial arrangement in 2 – Butene.
When two compounds having the same molecular formula, similar chemical structures and double bond possess different geometrical arrangements of the atoms or groups about the doubly bonded carbon atoms, the phenomenon is known as geometrical isomerism and such compounds are known as geometrical isomers.
When the two identical atoms or groups are on the same side of the double bond the isomer is called cis isomer; while if they are on the opposite sides, the isomer is called trans – isomer.
Cis-trans isomers have similar chemical properties but they differ in their physical properties.
Conditions for Geometrical Isomerism:
- There should be a double bond in the molecule.
- The two atoms or groups attached to each doubly bonded carbon atom should be different. Thus abC=Cab. abC=Ccd, abC=Cax can show cis-trans isomerism.
- Consider following examples
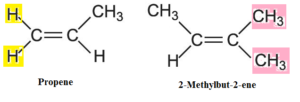
- In propene two hydrogen atoms (same atoms) are attached to one of the doubly bonded carbon atoms. Hence it can not show cis-trans isomerism.
- In 2-Methyl-but-2-ene, two methyl groups (same group) are attached to one of the doubly bonded carbon atoms. Hence it can not show cis-trans isomerism.
Examples of Geometrical Isomerism:


Comparision of Properties of Cis and Trans Isomers:
- cis-isomer is more polar than the trans isomer because in it the individual bond dipoles do not cancel each other. In trans-isomers, the individual bond dipoles almost cancel each other hence their dipole moment is almost zero.

- Due to greater polarity cis-isomers has a greater boiling point than its trans-isomer.
- Due to symmetry, the molecules of trans-isomers are closely packed and show a higher melting point than its cis isomer.
- trans-isomers are more stable than cis-isomers.
Cahn-Ingold-Prelog Priority Rules:
- For higher substituted stereoisomers, the concept of cis and trans isomers is not sufficient. Hence Cahn-Ingold and Prelog proposed priority rules using which higher substituted stereoisomers are expressed in a form called E-Z notation. If the two groups of higher priority are on the opposite sides of the double bond, then the double bond is assigned the configuration E (derived from German word entgegen means the opposite). If the two groups of higher priority are on the same side of the double bond, then the double bond is assigned the configuration Z (derived from German word zusammen means together). The rules of assigning priority are as follows.
- Locate the four atoms directly attached to the stereocenter (X). Assign priorities based on the atomic number to all four atoms. Priority 1 is assigned to the atom or group of highest atomic number, priority 4 to the lowest. Use the following sequence of elements arranged in decreasing order of atomic number for determining priority. I (Iodine) > Br (Bromine) > Cl (Chlorine) > F (Fluorine)> O (Oxygen)> N (Nitrogen) > C (Carbon) > T (Tritium) > D (Deuterium) > H (Hydrogen)

- If two or more atoms are identical (designated A and B ), Locate all the atoms directly attached to the identical atoms in questions (designated A-1, A-2, A-3 and B1, B-2, B-3). Assign priorities to all these atoms based on atomic number (1 is the highest priority, 3 the lowest). Compare the highest priority atoms, i.e. compare A-1 with B-1. If A-1 is a higher
priority atoms than B-1, then A is a higher priority than B. If A-1 and B-1 are the same atoms, then compare the second highest priority atoms directly bonded to A and B (A-2 with B-2); if A-2 is a higher priority atom than B-2, then A is higher
priority than B. If A-2 and B-2 are identical atoms, compare A-3 with B-3 and so on. - Multiple bonds are considered as an equivalent number of single bonded atoms.

- Example – 1: 1-Bromo-1-chloro-2-iodopropene:

Priority: I > Br > Cl > C
- In the first configuration I and Br the high priority groups are on opposite sides of the double bond, hence it is E configuration and the compound is named as (E)-1-Bromo-1-chloro-2-iodopropene.
- In the second configuration I and Br the high priority groups are on the same side of the double bond, hence it is Z configuration and the compound is named as (Z)-1-Bromo-1-chloro-2-iodopropene.
- Example – 2: 2-Iodo-2-pentene

Priority: I > CH3CH2 > CH3 > C
- In the first configuration I and CH3CH2 the high priority groups are on opposite sides of the double bond, hence it is E configuration and the compound is named as (E)-2-Iodo-2-pentene.
- In the second configuration I and CH3CH2 the high priority groups are on the same side of the double bond, hence it is Z configuration and the compound is named as (Z)-2-Iodo-2-pentene.
Structure of Ethylene (Ethene):
Hybridization of Carbon in Ethylene Molecule:
- Ethene is built from hydrogen atoms (1s1) and carbon atoms (1s22s22px12py1). Carbon atom undergoes sp2 hybridization. It has (1s22s22px12py1) configuration in its ground state. It promotes one of the electrons from 2s2 pair into the empty 2pz orbital. This state has (1s22s12px12py12pz1) configuration. One s and 2 p ( px and py) mix and form three sp2 hybridized orbitals. pz orbital do not take part in the hybridization. The three sp2 hybrid orbitals arrange themselves as far apart as possible – which is at 120° to each other in a plane. The remaining pz orbital is at right angles to them.
- Two sp2 hybridized orbitals of each carbon atoms undergo axial overlap with 1s orbital of two hydrogen atoms to form sigma (σ) bonds. Thus there are 4 C-H overlaps (sigma bonds). The remaining sp2 hybrid orbital of each carbon overlap axially to form a C-C bond (sigma bond). Unhybridized pz orbitals of the two carbons overlap laterally to form C-C (pi bond). Thus between two carbons, there is a double bond ( 1 sigma bond and another pi bond).

Dot and Dash Structure of Ethylene Molecule:
- In Ethene, each carbon atom utilizes two electrons in making two covalent bonds with two hydrogen atoms, and one electron in making one covalent bond with another carbon atom. The fourth electron of each carbon forms the double bond between the two carbon atoms. This is the conventional formula for Ethene.

Bond Lengths and Bond Angles in Ethylene Molecule:

Ball and Stick Model of Ethylene Molecule:

π Cloud of Ethylene Molecule:
