Many substances isolated from resins, balsams, and essential oils possessed a fragrant smell and were found to be different from aliphatic compounds in chemical behaviour. Such substances were called aromatic compounds (Greek Aroma – fragrant smell). All aromatic compounds do not have a fragrant smell while some aliphatic hydrocarbons have a fragrant smell. Hence the term arene is used for aromatic hydrocarbons. Nowadays the term aromatic is reserved for benzene and for all carbocyclic derivatives which resemble with benzene in chemical behaviour. Benzene like compounds are also t as benzenoid compounds. There are some compounds which resemble benzene in their chemical behaviour but do not contain benzene ring are called non-benzenoid compounds.
Aromatic Hydrocarbons:
Aromatic hydrocarbons contain a higher percentage of carbon than the corresponding aliphatic compounds and contain at least six carbon atoms. They are closed chain or cyclic compounds.
Benzene C6H6 is the simplest aromatic compound. It is regarded as the parent substance from which practically all the aromatic compounds are derived. Hence, aromatic chemistry is the chemistry of benzene and all the compounds structurally related to it.
Aromatic hydrocarbons are defined as cyclic carbon compounds which are related to benzene and at least contain one aromatic (benzene-like) nucleus. e.g. benzene, naphthalene etc.
The general formula of arenes is cnH2n – 6y, where y is the number of the benzene ring and n is not less than 6.
The benzene ring found in aromatic compounds have following structure. It is called the benzene nucleus.
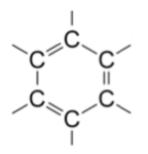
Characteristics of Aromatic Compounds:
Main characteristic features in which the aromatic compounds differ from aliphatic compounds are given below
- Aromatic compounds contain a higher percentage of carbon than the corresponding aliphatic compounds. Benzene C6H6 contains 2.3% of carbon while corresponding aliphatic compound hexane C6H14 contains 83.7% of carbon.
- Due to the higher percentage of carbon, aromatic compounds burn with sooty or smoky flame while aliphatic compounds burn with a luminous flame.
- Aromatic compounds are closed chain compounds whereas aliphatic compounds have open chain structures.
- The nucleus (ring of atoms) in aromatic compounds is highly stable.
- The aromatic compounds, though they contain double bonds, differ from alkenes, in fact, that they are quite stable, and do not undergo addition reactions easily. Aromatic compounds show substitution reactions like halogenation, nitration, and sulphonation.
- They give nitro derivatives when heated with concentrated nitric acid ‘(nitration). Aliphatic compounds do not form nitro derivatives easily.
- When treated with concentrated sulphuric acid, aromatic compounds undergo sulphonation which is not very common with aliphatic compounds.
- The hydroxy derivatives of aromatic compounds (phenols) are acidic in nature, while the hydroxy derivatives of aliphatic compounds (alcohols) are neutral.
- Chlorine and bromine form addition as well as substitution product with aromatic compounds.
- Aromatic amines undergo diazotisation while aliphatic amines do not undergo diazotisation.
- Aromatic halogen compounds are less reactive than alkyl (aliphatic) halides.
- Homologues of benzene get oxidized to benzoic acid, irrespective of the length of the chain.
- Friedel-Craft’s reaction, Perkin reaction, mercuration and chloromethylation are only shown by aromatic compounds.
Examples and Names of Some Arenes:
Monocyclic Aromatic Hydrocarbons:
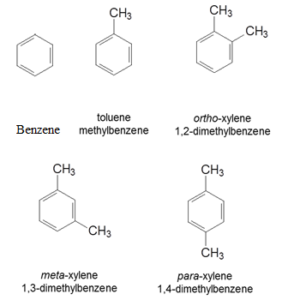
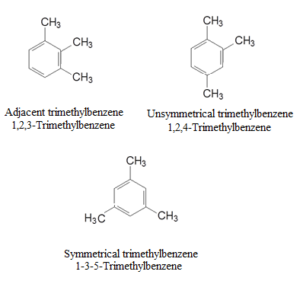

Polycyclic Aromatic Hydrocarbons (PAH):
- PAH’s (polycyclic aromatic hydrocarbons) are likely to be some of the most potent carcinogens (chemicals that cause cancer) known to science. They are found in coal and all fossil fuels.
- PAH containing isolated benzene rings

- PAH containing fused or condensed benzene rings

PAHs as Carcinogens:
PAH’s are likely to be some of the most potent carcinogens (chemicals that cause cancer) known to science. They are found in coal, all fossil fuels, (the main compounds in coal tar) and cigarette smoke. PAH’s are flat multi-ring structures that mimic PAH’s have the perfect structure to slip in between the steps of the DNA ladder (intercalation). DNA base pairs. PAH’s adjusts themselves in between the base pairs in DNA disrupting DNA replication and causing mutations in them. Cancer is caused by a series of DNA mutations.
PHAs have been shown to cause cancer in laboratory animals, but not in Humans. There are no “safe levels” defined, so PAH’s are largely overlooked in public health considerations. They are more dangerous than asbestos.
Sources of Aromatic Hydrocarbons:
Coal:
Carbonization or Coking of a Coal:
Carbonization of coal is also known as coking of coal. The process consists of thermal decomposition of coals either in the absence of air or in a controlled atmosphere to produce a carbonaceous residue known as coke.
Carbonization can be carried out at low temperature (450-750 °C)or high temperature (above 900 °C). Low-temperature carbonization is used to produce liquid fuels while high-temperature carbonization is used to produce gaseous products.
Coke (about 70%), Coal tar (4-5%), Ammonia liquor (8-10%) and coal gas (17%) are the main products of carbonization of coal.

Coke: Coke is a high-carbon product obtained by the destructive distillation of coal. Coke is greyish-black in colour and is a hard, porous solid. The amount of carbon content in coke is so high that it is said to be an almost-pure form of carbon. The burning of a coke produces very little smoke. The most common use of coke is as a domestic and industrial fuel. It is used in a blast furnace and to manufacture steel and many other materials.
Coal tar: It is a highly viscous black liquid with an unpleasant odour. It also has an extremely unpleasant smell. Coal tar contains benzene, toluene, xylenes, naphthalene, anthracene, water (neutral substances), phenol, cresols (acidic substances) and pyridine, quinoline (basic substances). Coal tar is widely used to manufacture paints, perfumes, synthetic dyes, photographic material, drugs, explosives, insecticides, pesticides, naphthalene balls, anti-dandruff and lice-repelling shampoos, soaps and ointments.
Coal gas: This is the gaseous product obtained by carbonization of coal. It is a highly flammable gas and its main constituent is methane. It is mainly used as a domestic and industrial fuel and also used for street lighting in old days.
Ammonical Liquor: Ammonia is used for making fertilizers such as ammonium sulphate, ammonium superphosphate etc.
Coal Tar Distillation:
Coal tar is obtained as a by-product during coking of coal. Coal tar pitch is a complex chemical mixture of phenols, cresols and xylenols (which together termed as tar acids), polycyclic aromatic hydrocarbons (PAH), and heterocyclic compounds. These constituents are separated by fractional distillation of the coal tar.
e purpose of tar distillation is to 1) dehydrate the tar in the dehydration column, 2) remove the pitch from dehydrated tar in pitch column and 3) separate tar oils in a fractionating column.
The different fractions obtained are
| Sr. | Main Fraction | Temperature Range | Constituents |
| 1 | Light oil or crude naptha | 353 – 443 K | Benzene, toluene, xylene, etc. |
| 2 | Middle oil or carbolic acid | 443-503 K | Phenol, naphthalene, pyridine, etc. |
| 3 | Heavy oil or creosote oil | 503 K – 543 K | Cresols, naphthalene, quinoline, etc. |
| 4 | Green oil or anthracene oil | 543-633 K | Anthracene, phenanthrene, etc. |
| 5 | Pitch | Residue | Most carbon |
Each fraction is redistilled for separation of the constituent and each constituent obtained is further distilled for purification.
Petroleum:
Petroleum is a mixture of different hydrocarbon molecules of different sizes and a different number of carbon atoms. Each constituent has its own boiling point. The fractional distillation is used to separate the constituent of petroleum (crude oil). The specific fractions obtained are:
Paraffines: C1-C4 (mainly Propane), C4-C12 (gasoline, petrol ether), C12-C15 (kerosene), C15-C25 (diesel), >C25 (wax, asphalt)
Cycloparaffines: Cyclohexane, Cyclopentane
Aromates: Benzene, Toluene, Ethylbenzene, Xylene

The alkanes obtained from the distillation of crude oil are heated over platinum-rhenium-alumina catalyst at 793 K at about 20 atmospheric pressure to obtain cyclic compounds.
n – Hexane → Cyclohexane → Benzene
n-Heptane → Methyl cyclohexane → Toluene
1,3 – Butadine + Ethene → cyclohexene → Benzene