In the last article, we have studied methods of preparation of alkenes. In this article, we shall study the reactions of alkenes
Stability of Alkenes:
The stability of alkenes is measured in terms of its heat of hydrogenation. The heat of hydrogenation of an alkene is defined as the amount of heat evolved when one mole of alkene is completely hydrogenated to form alkane. An alkene having the greater value of the heat of hydrogenation is less stable.
The more the number of alkyl groups attached to the doubly bonded carbon atoms, greater is the stability of the alkene. The greater stability is due to the fact that greater the number of alkyl groups attached to the doubly bonded carbon atoms, greater is the delocation of π electrons through hyperconjugation which leads to the stability of alkenes.
R2C=CR2 (4 alkyl groups) > R22C=CHR (3 alkyl groups)> R2C=CH2 (2 alkyl groups) > RCH=CHR (2 alkyl groups) > RCH=CH2 (1 alkyl group) > CH2=CH2 (No alkyl group).
Trans isomers (E configuration) are more stable than cis-isomers (Z configuration)
Reactivity of Alkenes:
Alkenes have carbon-carbon double bond. one of the bonds is sigma (σ) bond while another bond is pi (π) bond. The extent of sigma bond is more (due to axial overlapping) than that of a pi bond (due to sideways overlap). The bond energy of sigma bond is 83 Kcal/mol while that of the pi bond is 63 Kcal/mol. The electron density of pi bond is diffused and exposed more to an attacking electrophile.
The pi bond is under strain and tries to convert into two sigma bonds. Hence they attract electrophile easily. Hence alkenes undergo electrophilic addition reactions.
Reactions of Alkanes:
Unlike alkanes, alkenes are very reactive and they undergo addition reactions to form a saturated compound.
Addition Reactions:
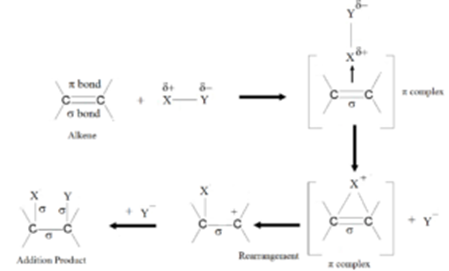
Mechanism of Electrophilic Addition:
Let us consider an attacking molecule XY, such that the part Y of the molecule is more electronegative than X. Being more electronegative than X, Y pulls the shared pair of electrons towards it. Hence the molecule can be represented as Xδ+Yδ-.
XY molecule attack on alkene and adds to the π electrons cloud forming a bond through Xδ+(electrophilic part). During this, a π complex is formed. The π complex undergoes rearrangement to form unstable carbocation which on the addition of Y– gives the addition product.
Hydrogenation of Alkenes or Addition of Dihydrogen:
When vapours of alkene are mixed with dihydrogen and are passed over catalyst like Nickel, Platinum or Palladium corresponding alkane is formed.
General Reaction:
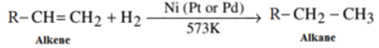
Example – 1: Hydrogenation of Ethene:
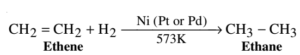
Example – 2: Hydrogenation of Propene:

- Pt and Pd catalyst are effective at room temperature. Alkenes cannot be hydrogenated by nascent Hydrogen.
- Hydrogenation is a very important process because it is used in the manufacturing of vanaspati from vegetable oils.
Halogenation of Alkenes:
Alkenes combine with halogen in presence of inert solvent CCl4 at room temperature readily to form alkylene dihalide. Iodine does not give this reaction.
General reaction:

Examples – 1: Action of Bromine on Ethane:

In such reactions, bromine (reddish brown colour) gets decolourised, and it is used as a test for alkenes (test for unsaturation).
Example – 2: Action of Bromine on Propene:

Action of Haloacids on Symmetric Alkenes:
The addition of halogen acids like HCl, HBr or HI to a compound containing multiple bond is known as hydrohalogenation. Alkenes react with halogen acid to form corresponding alkyl halide.
- The order of reactivity is HI > HBr > HCl.
- HCl reacts as per Markownikoff’s rule only.
General reaction:
R–CH=CH2 + HX → R–CH2–CH2X
Alkene Alkyl halide
Examples With Symmetric Alkenes:
Example – 1: Action of HBr on Ethene:
CH2=CH2 + HBr → CH3–CH2Br
Ethene Ethyl bromide
Example – 2: Action of HBr on But-2-ene:
CH3–CH=CH–CH3 + HBr → CH3–CH2–CHBr–CH3
But-2-ene 2-Bromobutane
Action of Haloacids on Unsymmetric Alkenes:
Markownikoff’s rule: When an unsymmetrical alkene is treated with an unsymmetrical reagent like HBr, then the negative part of, the reagent goes to that carbon atom which has less number of hydrogen atoms attached to it.
Example-1: Action of HBr on Propene:

Example-2: Action of HBr on But-1-ene:

Anti-Markownikoff’s rule (Peroxide effect or Kharasch effect): When an unsymmetrical alkene is treated with an unsymmetrical reagent like HBr in presence of a peroxide, then the negative part of, the reagent goes to that carbon atom which has more number of hydrogen atoms attached to it.
Example-1: Action of HBr on Propene in Presence of Peroxide:

Example-2: Action of HBr on But-1-ene in Presence of Peroxide:

Action of Water:
Alkenes add a molecule of water in the presence of mineral acids such as H2SO4. Addition takes place as per Makonikoff’s rule.
General Reaction:

Example – 1: Action of water on ethene:

Example – 2: Action of water on propene (Asd per Markownikoff’s rule):

Mechanism of Addition of Water to Alkene:

Addition of Hypohalous Acids:
Alkenes add hypohalous acid (HO-X) across the double bond to give halohydrins. The addition takes as per Markonikoff’s rule and OH acts as negative part of the reagent.
General Reaction:

Example – 1:

Example – 2:

Oxidation Reactions:
Combustion of Alkenes:
Alkenes are combustible and burn with luminous flame in air or oxygen to form carbon dioxide and water with evolution of large amount of heat.
CH2=CH2 + 3O2 → 3 CO2 + 2H2O ; ΔH° = -1411 kJ mol-1.
Oxidation with potassium permanganate:
With Cold and dilute KMnO4 solution:
This reaction is Baeyer’s test.
General Reaction:

Example – 1:

Example – 2:

Ozonolysis of Alkene:
Alkene on treatment with ozone in presence of organic solvents like CCl4 forms alkylene ozonide, which on hydrolysis in presence of reducing agent like zinc give aldehydes or ketones or a mixture of both. This reaction is called an ozonolysis.
Examples – 1: Action of Ozone on Ethene:

Examples – 2: Action of Ozone on Propene:

Uses of Alkanes:
- Ethene gas is used in the early ripening and storage of fruits and vegetables.
- Oxy-ethylene gas is used for cutting and welding metal.
- Ethene is used for the preparation of ethyl alcohol and acetaldehyde.
- Ethene is used in the preparation of Polythene and resins polymers.
- Ethene gas is used as an anesthetic.
- Ethene is used for the preparation of mustard gas.