Science > Chemistry > Introduction to Chemistry > Chemical Classification of Substances
In the last article, we have studied the significance of chemistry and its branches. In this article, we shall study the chemical classification of substances.
Matter and its Different States:
The matter is any substances that have mass and that occupies space. There are three states of matter.
- Solid: It has a definite shape and volume at given temperature and pressure.
- Liquid: It will take the shape of the container, thus has indefinite shape but has a definite volume.
- Gas: Gas has neither a definite shape nor a definite volume.
Note: Besides these three standard states, there are two more states called plasma state (Exists at very high temperature) and Bose-Einstein condensate (Exists at very cold condition).
Depending upon the chemical composition matter is classified into two types a) Pure substances and b) Mixtures.
Pure substances are further classified into elements (e.g. iron, oxygen) and compounds (e.g. sodium hydroxide, ammonia).This classification was given by a French chemist Lavoisier. While the mixtures are further classified into homogeneous mixtures (e.g. a common salt solution in water)and heterogeneous mixtures (e.g. muddy water).
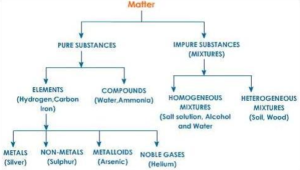
Pure Substances:
A pure substance is the one which is made up of molecules containing the same kind of atoms. Let us consider the case of pure water. In pure water, all molecules are made up of two hydrogens and one oxygen. Pure substances are further classified into elements and compounds.
All pure substances are homogeneous. But all homogeneous substances are not pure. For e.g. If we consider water its composition is same everywhere, thus it is homogeneous. If we take lime water its composition may be the same everywhere but it is not a pure substance. It is a mixture of water and lime juice.
Characteristics of the Pure Substances:
- They have fixed composition.
- They cannot be separated into simpler substances.
- They can only be changed in identity and properties.
- Their properties do not vary.
Elements:
An element may be defined as a pure substance which can neither be decomposed into nor built from simpler substances by any physical or chemical method. Now atom is considered a fundamental unit of matter. Hence an element may be defined as a pure substance which is made up of one kind of atom.
This definition fails to explain modern techniques and processes like nuclear fission, nuclear fusion and artificial transmutations in nuclear chemistry.
There are presently 118 different elements known. Every element has been given a definite name and a definite symbol. A symbol is a small abbreviation to represent a full and lengthy name of the element. For example: element Nitrogen is represented by the symbol N, element Calcium is represented by the symbol Ca, etc.
Out of 118 elements known, 89 have been isolated from natural sources and the remaining have been prepared by artificial means in laboratories. These elements are called man-made elements. Only ten of naturally occurring elements make up 99% mass of the earth’s crust, oceans, and atmospheres.
Characteristics of Elements:
- They cannot be divided into simpler substances.
- They cannot be built from simpler substances.
- They can only be changed in identity and properties.
- Their properties do not vary.
Classification of Elements:
Based on nature, elements are classified into three types: a) Metals, b) Non-metals, and c) Metalloids.
Metals::
These are generally solids (exceptions mercury and gallium). Examples: Copper, iron, aluminium, zinc, etc.
They have characteristics such as high density, high melting and boiling points, hardness, malleability, high tensile strength, lustre, and the ability to conduct heat and electricity. They react with mineral acids and liberate hydrogen. They form basic oxides. They form non-volatile hydrides if combine with hydrogen.
Note that lithium, sodium, potassium possess low density.
Non-metals:
These are generally non-lustrous. Examples: Sulphur, phosphorous, nitrogen, hydrogen. Six of the non-metals viz: carbon, boron, phosphorous, sulphur, selenium, and iodine are solids. Bromine is the only liquid non-metal at room temperature and pressure. The remaining non-metals: nitrogen, oxygen, fluorine, chlorine, hydrogen, helium, argon, neon, krypton, xenon, and radon are gases.
Generally, they are brittle, non-lustrous, have low melting and boiling points, non-conductors of heat and electricity. They form acidic or neutral oxides. They do not react with mineral acids to liberate hydrogen. They are capable of forming volatile hydrides.
Note that hydrogen and graphite are good conductors of electricity.
Metalloids:
These elements have characteristics common to metals as well as non-metals. Examples: Arsenic, tin, bismuth, antimony, silicon, tellurium.
Notes:
- Metals rarely combine with one other.
- Non-metals combine with one another to form compounds.
- Metals and Non-metals commonly combine with each other to form compounds.
Atomicity of an Element:
The number of atoms present in a molecule of an element is called its atomicity. Based on their atomicity they are classified as
- Monoatomic elements: e.g. Silver (Ag), Gold (Au), Aluminium (Al), Helium (He), Neon(Ne), Boron (B), Carbon (C).
- Diatomic elements: Hydrogen (H2), Chlorine (Cl2), Oxygen (O2).
- Polyatomic elements: Ozone (O3), Phosphorous (P4), Sulphur (S8).
Differences Between Metals and Non-Metals:
| Metals | Non-metals |
| These are generally solids. (Exceptions: mercury and gallium, they are in the liquid state at normal temperature and pressure). | They are obtained in solid or gaseous states. (Exception: Bromine, it is in the liquid state at normal temperature and pressure). |
| They have high densities (Exceptions: lithium, sodium, potassium possess low density) | They have low densities |
| They are good conductor of heat and electricity. | They are bad conductor of heat and electricity. (Exceptions: Hydrogen and graphite are good conductor of electricity) |
| They react with mineral acids and liberate hydrogen. | They do not react with mineral acids. |
| They form basic oxides. | They form acidic or amphoteric oxides. |
| They form non-volatile hydrides if combine with hydrogen. | They form volatile hydrides if combine with hydrogen. |
| Examples: Aluminium, Copper, etc. | Examples: Carbon, sulphur, etc. |
Compounds:
A compound may be defined as a pure substance which can be decomposed into simpler substances by some suitable chemical method. A compound is formed by the combination of two or more elements in a definite proportion of a mass.
Examples: Water (H2O) is a compound of hydrogen and oxygen in the ratio 1:8 by mass respectively. Carbon dioxide (CO2) is a compound of carbon and oxygen in the ratio 3:8 by mass respectively.
Characteristics of Compounds:
- The constituents of a compound are always present in a fixed ratio by mass.
- A compound is always homogeneous in nature.
- The properties of the compound are different from those of its constituent elements.
- The constituents of a compound cannot be separated by simple mechanical means. Energy in the form of light, heat or electricity is required to separate them.
- Compounds are formed as a result of a chemical change.
- The formation of a compound is always accompanied by absorption or evolution of heat, light, or electrical energy.
- Compounds in their purest form have sharp boiling and melting point.
Types of Compounds:
Organic Compounds: The compounds obtained from living sources (organisms) are called organic compounds. Nowadays the term organic compounds refer to compounds of carbon and hydrogen (Hydrocarbons) and their derivatives.
Inorganic Compounds: The compounds obtained from non-living sources such as rocks and minerals are called inorganic compounds. Compounds of all elements except hydrocarbons and their derivatives are included in this type of compound.
It is to be noted that the number of organic compounds is very large compared to inorganic compounds.
Mixtures:
Mixtures can be defined as substances which are made up of two or more pure substances. They can possess variable composition and can be separated into constituent components by some suitable physical means.
Example: Crude oil, air
Classification of Mixtures:
Mixtures are classified into two types
Homogeneous Mixtures: Homogeneous mixtures are the mixtures which have the same composition throughout. These mixtures are also known as solutions. The constituents of homogeneous mixtures are distributed uniformly throughout. The constituents of such mixtures cannot be seen even under the microscope. Examples: Air, gasoline, sea water, stainless steel, brass, coloured glass etc.
Heterogeneous Mixtures: Heterogeneous mixtures are the mixtures which have different composition at different parts. The constituents of heterogeneous mixtures are not distributed uniformly throughout. The constituents of such mixtures can be seen even by naked eyes or with the help of a microscope. Examples: Mixture of iron filings and sulphur, muddy water, a mixture of sand and sugar etc.
Difference Between Homogeneous Mixtures and Heterogeneous Mixtures:
| Homogeneous Mixtures | Heterogeneous Mixtures |
| Homogeneous mixtures are the mixtures which have the same composition throughout. | Heterogeneous mixtures are the mixtures which have different composition at different parts. |
| The constituents of homogeneous mixtures are distributed uniformly throughout. | The constituents of heterogeneous mixtures are not distributed uniformly throughout. |
| The constituents of such mixtures cannot be seen by naked eyes and even not with the help of a microscope. | The constituents of such mixtures can be seen even by naked eyes or with the help of a microscope. |
| The Constituents of such mixtures cannot be separated easily. | The Constituents of such mixtures can be separated very easily |
| Examples: Air, gasoline, sea water, stainless steel, brass, coloured glass etc. | Examples: Mixture of iron filings and sulphur, muddy water, a mixture of sand and sugar etc. |
Characteristics of Mixtures:
- The constituents of the mixture may be present in any ratio.
- Mixtures may or may not be homogeneous in nature.
- The properties of constituents remain the same even in mixture form.
- The constituents of a mixture can be separated by simple mechanical or physical means.
- Mixtures are formed as a result of a physical change.6. The formation of a mixture is not accompanied by absorption or evolution of heat, light, or electrical energy.
- Mixtures don’t have sharp boiling and melting point.
In the next article, we shall study different methods of separation of constituents of a mixture.
Previous Topic: Significance of Chemistry
Next Topic: Methods of Separation of Mixtures
