Science > Chemistry > States of Matter > Gaseous State
In this article, we shall study the general characteristics of the gaseous state and the measurable properties of gases.
General Characteristics of Gaseous State:
- A gas has neither a definite shape nor a definite volume. They acquire the volume and shape of the container in which they are kept.
- A gas is capable of expanding to any limit and is capable of occupying whole available space.
- Gas is highly compressible. As the pressure on the gas is increased its volume decreases.
- Gases have comparatively less density w.r.t. solids and liquids.
- Gases have two specific heats viz. specific heat at constant pressure and specific heat at constant volume.
- In the gaseous state, the intermolecular forces of attraction are very weak i.e. almost zero.
- The molecules of a gas are in continuous random motion.
- Gases have the capacity to diffuse with each other irrespective of the difference in their densities.
- Gases have low viscosity.
- A gas exerts pressure on the walls of the container in which it is kept.
- All the gases obey certain laws known as gas laws. These relat5ions or laws are based on experimental results.
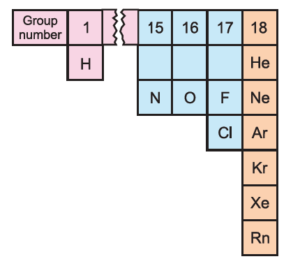
Note: Eleven out of all known elements exist in the gaseous state under normal atmospheric conditions of temperature (25° C) and pressure (1 bar) are as shown below.
Measurable Properties of a Gases:
The measurable properties used to describe the physical state of a gas are (a) mass, (b) volume (c) pressure and (d) temperature.
Mass:
It is given directly or in terms of moles of gas. It is measured in grams or kilograms. Generally, the mass of a gas is expressed in terms of moles.
Number of moles of a gas = Given mass of the gas/Molecular mass of the gas = m/M
Molecular masses of some gases are hydrogen (2), oxygen (32), nitrogen (28), carbon dioxide (44), ammonia (17), methane (16), and chlorine (71)
Measurement of Mass of a Gas:
The mass of the container with enclosed gas is measured. Then the container is completely emptied and the mass of the empty container is measured. The difference between the two masses gives the mass of the gas in the container.
Conversions:
| From | Into | Multiplying Factor |
| kg | g | × 10³ |
| g | kg | × 10-3 |
| mg | g | × 10-3 |
Volume:
It means space occupied by the gas. It is measured in cubic centimeters, millilitres (mL), litres (L), dm3, m3. In the case of gas, the volume of the gas is equal to the volume of the container containing it.
Measurement of Volume of a gas:
The gas acquires volume and shape of the container in which they are kept. Hence the volume of the container is taken as the volume of the gas.
Conversions:
| From | Into | Multiplying Factor |
| L | dm³ | × 1 |
| dm³ | m³ | × 10-3 |
| cm³ | m³ | × 10-6 |
| mL or cc | L | × 10-3 |
| L | m³ | × 10-3 |
Temperature:
The temperature of a gas is a measure of the quantity of energy possessed by the gas molecules. It is measured in °C (centigrade or celsius) or K (Kelvin).
Measurement of Temperature of a gas:
The temperature may be defined as the degree of hotness. It is measured by a device called thermometer. The common thermometer is a mercury thermometer.
Various Temperature Scales:
Celsius Scale (° C):
In this scale, the melting point of ice at one-atmosphere pressure and at mean sea level is taken as the lower reference point and consider as 0° C. While boiling point of water at one-atmosphere pressure and at mean sea level is taken as an upper reference point and consider as 100° C. The range between the two reference points is divided into 100 equal parts and each part is called 1° C (one-degree celsius). This scale is also called a centigrade scale.
A lower limit 0° C is considered arbitrary, this scale can be extended to indicate negative temperatures also. The temperature below -273.15° C is not possible.
Farenheit Scale (° F):
In this scale, the melting point of ice at one-atmosphere pressure and at mean sea level is taken as the lower reference point and consider as 32° F. While boiling point of water at one-atmosphere pressure and at mean sea level is taken as the upper reference point and consider as 212° F. The range between the two reference points is divided into 180 equal parts and each part is called 1° F (one-degree farenheit). Nowadays, this scale is not in use.
Kelvin Scale (K):
In this scale, the lowest possible temperature -273.15° C is taken as a lower reference point. This temperature is called absolute zero. The division of 1 K is equal to 1° C. The unit of temperature in the kelvin scale is K (kelvin) and is considered as the fundamental unit in S.I. system of units.
Conversion of Temperature in Different Scales:
Celsius scale to Kelvin scale ° C + 273 = K
Kelvin scale to celsius scale K – 273 = ° C
Pressure:
It means the total force exerted by the gas molecules per unit area of the container walls due to their collision on the same walls. It is measured In terms of the height of the mercury column in centimetres or milimetres or Nm-2 or Pa (pascal).
The gas molecules are in random motion. During random motion, they collide with each other and with the walls of the container. During the collision with walls of the container, they exert outward force on the walls of the container. The outward force per unit area of the walls is called the pressure of the gas.
The S.I. unit of pressure is Nm-2 (newton per square metre). It is called Pa (pascal). Other units of pressure used are atm (atmosphere), bar, and in terms of the height of the mercury column.
Atmospheric Pressure:
A thick blanket of air around the earth is called atmosphere. Molecules of various gases present in the atmosphere are under constant pull of gravitational force of the earth. Thus the weight of air column exerts pressure on the surface of the earth. The force experienced by any area of the earth exposed to the atmosphere is equal to the height of the column of air above it. This force per unit area of the earth is called the atmospheric pressure. The value of atmospheric pressure is not constant over the place. It varies with location, temperature and weather conditions. The atmospheric pressure is very large, but living beings are not getting crushed under it because the pressure inside the body of living beings is equal to that of atmospheric pressure.
Atmospheric pressure is measured by a simple device called barometer. It consists of a glass tube of about 1 m long, closed at one end filled with mercury and inverted in an open vessel. The level of mercury adjusts itself in the tube to balance the atmospheric pressure. At sea level, the height of the mercury column is approximately 76 cm above the mercury level in the open vessel.
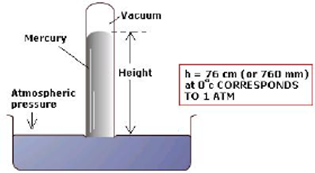
A standard atmospheric pressure (1 atm) is the pressure exerted by exactly 76 cm of mercury column at 0° C (273.15 K) measured at sea level where standard gravity is 9.806 ms-2, with the density of mercury being 13.596 g cm-3. mm of Hg unit is also called torr. The empty space (vacuum) above the mercury column in the tube is called Torricelli’s space.
Measurement of Pressure of a Gas:
The pressure of a gas is measured using a simple apparatus called mercury manometer or pre-calibrated pressure gauges. Open end manometers are suited for measuring pressure equal to or greater than the atmospheric pressure. While closed end manometer are suited for measure pressure below the atmospheric pressure. In closed end manometer, the difference in the levels of mercury in the two limbs gives the pressure of the gas.
In open end manometer, the difference in the levels of mercury in the two limbs gives gauge pressure of the gas.
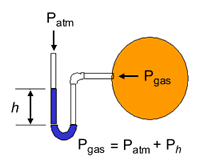
There are three possibilities in the measurement
- Levels of Hg is same in both the limbs
Gas Pressure = Patm
- Level of Hg in open limb is more than that connected to the gas container
Gas Pressure = Patm + Gauge Pressure
- Level of Hg in open limb is lower than that connected to the gas container
Gas Pressure = Patm – Gauge Pressure.
Conversions:
| From | Into | Multiplying Factor |
| kPa | Pa or Nm-2 | × 10³ |
| bar | Pa or Nm-2 | × 105 |
| atm | Pa or Nm-2 | 1.013 × 105 |
| mm of Hg | atm | ÷ 760 |
| cm of Hg | atm | ÷ 76 |
| mm of Hg | Pa or Nm-2 | × 13.6 × 9.8 |
| cm of Hg | Pa or Nm-2 | × 136 × 9.8 |
Numerical Problems:
Example – 01:
A manometer is connected to a gas containing bulb. The open arm reads 43.7 cm whereas the arm connected to the bulb reads 15.6 cm. If barometric pressure is 743 mm Hg. What is the pressure of the gas in the bulb in bar.
Solution:
Difference in mercury level of two arms = 43.7 cm – 15.6 cm = 28. 1 cm
Hence gauge pressure = 28. 1 cm of Hg = 281 mm of Hg
Absolute Pressure = Gauge pressure + Atmospheric pressure = 743 mm + 281 mm = 1024 mm
= 1024 mm/ 760 mm = 1.347 atm = 1.347 × 1.013 = 1.365 bar
Example – 02:
The side arm of an open-end mercury manometer is attached to a gaseous system. The level of mercury in the arm attached to the system is 125 mm lower than the open end arm. Is it higher than the atmospheric pressure or lower? What is the pressure of the system if the atmospheric pressure is 760 mm of Hg?
Solution:
The level of mercury attached to the system is lower than the level in the open arm. Hence pressure of the gas is more than the atmospheric pressure.
Gauge Pressure = 125 mm of Hg
Pressure of system = Gauge Pressure + Atmospheric Pressure = 125 mm + 760 mm = 885 mm of Hg
Example – 03:
Reading in mercury manometer closed end arm is 100 mm and in the arm attached to the system is 70 mm. What is the pressure of the system?
Solution:
System Pressure = Gauge Pressure = Difference in mercury level = 100 mm – 70 mm = 30 mm of Hg
Standard Temperature and Pressure (STP) and Normal Temperature and Pressure (NTP):
The volume of a given mass of a gas depends on its pressure and temperature. Hence we have to specify the pressure and temperature of the gas when specifying its volume. When solving problems on the gaseous state, the condition of STP and NTP means
P = 1 atm = 1.013 × 105 Pa, T = 273.15 K