Science > Chemistry > Physical Chemistry > Nature of Chemical Bond > Hybridization of Orbitals
In this article, we shall study the concept of hybridization of orbitals. This concept overcomes the limitations of valence bond theory.
Need for Hybridization Concept:
A simple approach based on the overlap of s and p orbitals can be applied to many molecules, but it fails to explain the formation of compounds of Beryllium (Be), Boron (B), and carbon (C). The electronic configurations of Be, B, and C in the ground state are as follows.
The atoms of Beryllium Be (Z = 4) Electronic configuration is 1s2 2s2 (With no unpaired electrons).

The atom of Boron B (Z = 5) Electronic configuration is 1s2 2s2 2p1 (With one unpaired electron).
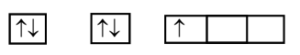
The atom of Carbon C (Z = 6) Electronic configuration is 1s2 2s2 2p2 (With two unpaired electrons).

According to valence bond theory valency of an element depends on a number of unpaired electrons in the orbitals. Thus Beryllium, Boron, and Carbon should be zero-valent, monovalent and divalent respectively. However, these elements form the compounds having valency 2, 3, and 4 respectively. Valence bond theory fails to explain this phenomenon. This is explained by hybridization.
Valence Bond Theory fails to explain the observed geometry of the molecules of water and ammonia e.g. in the formation of H2O molecule, the H – O – H bond angle should be 90°. But the measured bond angle is 104.3° and the molecule is V-Shaped. Valence bond theory failed to explain this change. To explain The equivalence of bonds we have to use the concept of a process of mixing and recasting of atomic orbitals. For e.g. all the four C-H bonds in methane molecule are equivalent in terms of strength, energy, etc. It can be explained on the basis of hybridization.
Note: The above paragraphs give limitations of the valence bond theory.
Hybridization:
Mixing and recasting or orbitals of an atom (same atom) with nearly equal energy to form new equivalent orbitals with maximum symmetry and definite orientation in space is called hybridization.
Steps Involved in Hybridization:
Step -1: Formation of excited state:
The atom in the ground state takes up some energy and goes to the excited state. In this process, usually, a pair of electrons in lower energy orbital is split up and one of the electron from this pair is transferred to some empty slightly higher but almost equal energy orbital. Thus the excited state has a larger number of half-filled orbitals. This new number of half-filled orbitals decides the number of covalent bonds an atom can form.

Step = 2: Mixing and recasting of atomic orbitals:
The orbitals of nearly the same energy in an excited state now hybridize i.e. unite and redistribute themselves giving hybrid orbitals of the same energy and definite orientation in space. Mixing and recasting or orbitals of an atom(same atom) with nearly equal energy to form new equivalent orbitals with maximum symmetry and definite orientation in space is called hybridization.
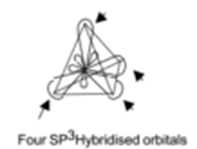
One 2-s orbital and three p- orbitals mix together and recast themselves to form new four sp3 hybridized orbitals.

Step – 3: Proper Orientation of the Hybrid Orbitals in Space:
The hybrid orbitals then get arranged in space in such a way to minimize mutual repulsion. Each hybrid orbital is more concentrated on one side of the nucleus. Due to this greater overlap is achieved and a stronger bond is formed.
Thus in carbon, the four hybrid sp3 orbitals arrange themselves at four corners of a tetrahedron to minimize mutual repulsion.

Essential Condition for Hybridization:
The orbitals participating in hybridization should have nearly the same energy. Thus in the formation of methane, the 2s and 2p orbitals of carbon have nearly the same energies, so that the recasting of orbitals is possible. But hybridization of 2s and 3p is not possible because there is much difference between their energies.
Characteristics or Rules of hybridization:
- Atomic orbitals undergoing hybridization should belong to the same atom or ion.
- Atomic orbitals participating in hybridization should have nearly the same energy. Thus 2s and 2p can hybridize, 3s and 3p can also hybridize, but 2s and 3p cannot.
- The total number of hybrid orbitals formed is equal to the number of atomic orbitals involved in the hybridization process.
- All hybrid orbitals are identical with respect to energy and directional character.
- The hybrid orbitals may differ from one other in their orientations.
- The shape of the hybrid orbitals is different from that of the original atomic orbital.
- Similar to atomic orbitals, each hybrid orbital can have a maximum of two electrons.
- The hybrid orbitals are concentrated in one particular direction to achieve greater overlapping.
- The hybrid orbitals have maximum symmetry and definite orientation in space so that the mutual force of repulsion of electrons is avoided.
Types of Hybridization and Geometry of Molecules:
The hybridization involving s and p orbitals are of the following three types:
- Tetrahedral or sp3 hybridization e.g. CH4, NH3, H2O
- Trigonal or sp2 e.g. BF3, C2H4.
- Diagonal or sp hybridization e.g. BeF2, C2H2
Their names indicate the orientation of the orbitals in space and the designation (sp2, sp3, etc) indicates the number and types of atomic orbitals involved in hybridization.
One reply on “Hybridization of Orbitals”
Loved the explanation of hybridization