The electrostatic force of attraction between the oppositely charged ions is called the ionic bond or electrovalent bond.
This bond is formed between two atoms whose electronegativity difference is large and they can attain octets by the complete transfer of one or more electrons from one atom to another. The atom which loses electrons is called an electropositive element and losing electron it forms a positive ion called a cation. The atom which acquires electrons is called an electronegative element and accepting electron it forms a negative ion called an anion. Generally, metals are electropositive and nonmetals are electronegative. Thus an ionic bond is formed between metallic and non-metallic atom. The oppositely charged formed ions are bound together by electrostatic attraction between them.
Thus an ionic or electrovalent bond is defined as a bond between two atoms is the electrostatic force of attraction which holds together the ions of combining atoms formed by the complete transfer of one or more electrons from the electropositive to the electronegative atom.
Examples of Ionic Bond:
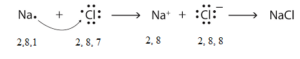
Formation of Sodium chloride (NaCl)
Electronic configuration of sodium Na (Z = 11) is 1s2 2s2 2p6 3s1 i.e. (2, 8, 1) while that of chlorine Cl (Z = 17) is 1s2 2s2 2p6 3s2 3p5 i.e. (2, 8, 7). Sodium has one electron in its valence shell. It can acquire stable configuration (2, 8) of nearest inert gas (Ne) by losing one electron and forming a positive ion (cation) of sodium. Chlorine has 7 electrons in its valence shell. It can acquire stable configuration (2, 8, 8) of nearest inert gas (Ar) by gaining one electron (lost by sodium) and forming a negative ion (anion) of chlorine.
The positive ion of sodium and negative ion of chlorine link together by a strong electrostatic force of attraction. Its representation is [Na+][Cl–]
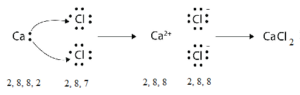
Formation of Calcium chloride (CaCl2):
Electronic configuration of sodium Ca (Z = 20) is 1s2 2s2 2p6 3s2 3p63 4s2 i.e. (2, 8, 8, 2) while that of chlorine Cl (Z = 17) is 1s2 2s2 2p6 3s2 3p5 i.e. (2, 8, 7). Calcium has two electrons in its valence shell. It can acquire stable configuration (2, 8, 8) of nearest inert gas (Ar) by losing two electrons and forming a positive ion (cation) of calcium with two unit positive charge. Chlorine has 7 electrons in its valence shell. It can acquire stable configuration (2, 8, 8) of nearest inert gas (Ar) by gaining one electron (lost by calcium) and forming a negative ion (anion) of chlorine. Two electrons lost by calcium are acquired by two chlorine atoms.
The positive ion of calcium and two negative ions of chlorine link together by a strong electrostatic force of attraction. Its representation is [Cu2+][Cl–]2
Formation of Lithium oxide (Li2O):
Electronic configuration of lithium Li (Z = 3) is 1s2 2s1 i.e. (2, 1) while that of oxygen O (Z = 8) is 1s2 2s2 2p4 i.e. (2, 6). Lithium has one electron in its valence shell. It can acquire stable configuration (2) of nearest inert gas (He) by losing one electron and forming a positive ion (cation) of lithium with unit positive charge. Oxygen has 6 electrons in its valence shell. It can acquire stable configuration (2, 8) of nearest inert gas (Ne) by gaining two electrons (lost one each by two lithium atoms) and forming a negative ion (anion) of oxygen with two negative charges. Two electrons lost by two lithium atoms are acquired by one oxygen atom.
Two positive ions of lithium and one negative ion of oxygen link together by a strong electrostatic force of attraction. Its representation is [Li+]2[O2-]
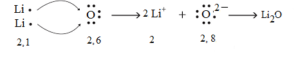
Formation of Magnesium oxide (MgO):
Electronic configuration of magnesium Mg (Z = 12) is 1s2 2s2 2p6 3s2 i.e. (2, 8, 2) while that of oxygen O (Z = 8) is 1s2 2s2 2p4 i.e. (2, 6). Magnesium has two electrons in its valence shell. It can acquire stable configuration (2, 8) of nearest inert gas (Ne) by losing two electrons and forming a positive ion (cation) of magnesium with two unit positive charge. Oxygen has 6 electrons in its valence shell. It can acquire stable configuration (2, 8) of nearest inert gas (Ne) by gaining two electrons (lost by magnesium atom) and forming a negative ion (anion) of oxygen with two negative charges. Two electrons lost by magnesium atom are acquired by the oxygen atom.
One positive ion of magnesium and one negative ion of oxygen link together by a strong electrostatic force of attraction. Its representation is [Mg2+][O2-]

Formation of Magnesium oxide (Na2S):
Electronic configuration of sodium Na (Z = 11) is 1s2 2s2 2p6 3s1 i.e. (2, 8, 1) while that of sulphur Cl (Z = 16) is 1s2 2s2 2p6 3s2 3p4 i.e. (2, 8, 6). Sodium has one electron in its valence shell. It can acquire stable configuration (2, 8) of nearest inert gas (Ne) by losing one electron and forming a positive ion (cation) of sodium. Sulphur has 6 electrons in its valence shell. It can acquire stable configuration (2, 8, 8) of nearest inert gas (Ar) by gaining two electrons (lost by two sodium atoms) and forming a negative ion (anion) of sulphur. Two electrons lost by two sodium atoms are acquired by one sulphur atom.
Two positive ions of sodium and one negative ion ofsulphur link together by a strong electrostatic force of attraction. Its representation is [Na+]2[S2-]
Ionic Bond and Periodic Table:
Electronegativities of Elements of Periodic Table

The number of electrons that aan atom gains or loses during the formation of an ionic bond is called electrovalency. Thus electrovalency is equal to the number of electrons lost by electropositive atom to form the positive ion and is equal to the number of electrons gained by electronegative atom to form the negative ion.
The tendency of an atom to gain or lose electron depends on its position in the periodic table. The elements of group 1 and 2 are highly electropositive and have very low ionization energies. Thus they prefer to form positive ions (cations) by losing electrons. The elements of group 15, 16 and 17 are highly electronegative and have very high ionization energies. Thus they prefer to form negative ions (anions) by gaining electrons.
If electro valencies of combining elements are known, then their empirical formula can be written very easily. The electrovalency of magnesium (Mg) is 2 and that of fluorine (F) is a. Hence the formula of the compound formed is MgF2.