Science > Chemistry > Laws of Chemical Combinations > Law of Reciprocal Proportions
In the previous article, we have studied the law of multiple proportions. In this article, we shall study the law of reciprocal proportions. The law of reciprocal proportions was given by German chemist Ritcher in 1792.

Statement:
The weights of two or more different elements which separately combine with a fixed weight of another element are either the same as, or simple multiples of, the weights of these elements when they combine among themselves.
Illustration 1:
Consider three compounds methane, carbon dioxide and water.
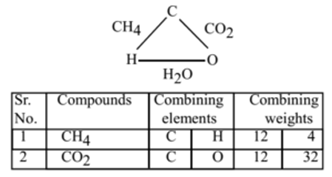
Hydrogen and oxygen react with carbon separately and forms methane and carbon dioxide respectively.
The ratio of different weights of hydrogen (4) and oxygen (32) are combining with fixed weight of carbon (12) is 4:32 i.e. 1:8.
Now hydrogen and oxygen combine to form water (H2O)in which the ratio of the weight of hydrogen to that of oxygen is 2:16 i.e. 1:8.
This ratio is the same as that of the first ratio obtained. Thus the law of reciprocal proportion is illustrated.
Illustration 2:
Consider three compounds phosphorous trihydride, phosphorous tri chloride and hydrogen chloride.

Hydrogen and chlorine react with phosphorous separately and forms phosphorous trihydride and phosphorous tri chloride respectively.
The ratio of different weights of hydrogen (3) and chlorine (106.5) are combining with fixed weight of phosphorous (31) is 3 : 106.5 i.e. 1 : 35.5
Now hydrogen and chlorine combine to form hydrogen chloride in which the ratio of the weight of hydrogen to that of chlorine is 1:35.5. This ratio is the same as that of the first ratio obtained. Thus the law of reciprocal proportion is illustrated.
Limitations of the Law of Reciprocal Proportions:
- The existence of isotopes of the element causes discrepancies similar to that observed In the law of constant proportions. Hence the same isotope or mixture of isotope should be used throughout the preparation of a series of compounds.
- Since there are few elements which will combine with the third element and also combine with each other. Thus the law is applicable to very few elements exhibiting the said property.
Explanation of the Law of Reciprocal Proportions on the Basis of Dalton’s Atomic Theory:
According to Dalton’s atomic theory, all the atoms of the same element are identical and the compounds are formed by the combination of atoms of different elements in a simple ratio of whole numbers. Therefore, the weights of the elements combining with a fixed weight of another element should bear a simple ratio to the ratio of the weights of the elements when they combine with each other. This explains the law of reciprocal proportion.
Numerical Problems:
Example – 01:
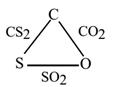
CO2 contains 27.27% of carbon, CS2 contains 15.79% of carbon and SO2 contains 50% of sulphur. Show that the data illustrates the law of reciprocal proportions.
Solution:

Consider CO2
% of carbon = 27.27
% of oxygen = 100 – 27.27 = 72.73
27.27 g of carbon combines with 72.73 g of oxygen.
Hence, 1 g of carbon combines with 72.73 / 27.27= 2.67 g of oxygen.
Consider CS2
% of carbon = 15.79
% of sulphur = 100 – 15.79 = 84.21
15.79 g of carbon combines with 84.21 g of sulphur.
Hence, 1 g of carbon combines with 84.21 / 15.79= 5.33 g of sulphur.
The ratio of different masses of sulphur and oxygen combining with fixed mass of carbon is 5.33 : 2.67
i.e. 2 : 1. ………………….. (Ratio 1)
Consider SO2
% of sulphur = 50
% of oxygen = 100 – 50 = 50
50 g of sulphur combines with 50 g of oxygen.
The ratio of mass of sulphur to that of oxygen is 50 : 50
i.e. 1 : 1 ……………… (Ratio 2)
The second ratio is a simple whole-number is multiple of the first ratio. Hence the data illustrate the law of reciprocal proportions.
Example – 02:
CuS contains 66.6 % of copper, CuO contains 79.9% of copper and SO3 contains 40 % of sulphur. Show that the data illustrates the law of reciprocal proportions.
Solution:

Consider CuS
% of copper = 66.6
% of sulphur = 100 – 66.6 = 33.4
66.6 g of copper combines with 33.4 g of sulphur.
Hence, 1 g of copper combines with 33.4 / 66.6= 0.5 g of sulphur.
Consider CuO
% of copper = 79.9
% of oxygen = 100 – 79.9 = 20.1
79.9 g of copper combines with 20.1 g of oxygen.
Hence 1 g of copper combines with 20.1 / 79.9= 0.25 g of oxygen.
The ratio of different masses of sulphur and oxygen combining with fixed mass of copper is 0.5 : 0.25
i.e. 2 : 1. ………………….. (Ratio 1)
Consider SO3
% of sulphur = 40
% of oxygen = 100 – 40 = 60
40 g of sulphur combines with 60 g of oxygen.
The ratio of the mass of sulphur to that of oxygen is 40 : 60
i.e. 2 : 3 ……………… (Ratio 2)
The second ratio is a simple whole-number multiple of the first ratio. Hence the data illustrate the law of reciprocal proportions.
Example – 03:
Aluminium carbide contains 75% of aluminium, aluminium oxide contains 52.9 % of aluminium and carbon dioxide contains 27.27 % of carbon. Show that the data illustrates the law of reciprocal proportions.
Solution:

Consider AlC3
% of aluminium = 75
% of carbon = 100 – 75 = 25
75 g of aluminium combines with 25 g of carbon.
Hence 1 g of aluminium combines with 2 5 / 7 5= 0.33 g of carbon.
Consider Al2O3
% of aluminium = 52.9
% of oxygen = 100 – 52.9 = 47.1
52.9 g of aluminum combines with 47.1 g of oxygen.
Hence 1 g of aluminium combines with 47.1 / 52.9= 0.89 g of oxygen.
The ratio of different masses of sulphur and oxygen combining with fixed mass of copper is 0.33 : 0.89
i.e. 1 : 3. ………………….. (Ratio 1)
Consider CO2
% of carbon = 27.27
% of oxygen = 100 – 27.27 = 72.73
27.27 g of carbon combines with 72.73 g of oxygen
the ratio of the mass of copper to that of oxygen is 27.27 : 72.73
i.e. 1 : 3 ……………… (Ratio 2)
The second ratio is a simple whole-number multiple of the first ratio (actually the same). Hence the data. illustrate the law of reciprocal proportions.
Example – 04:
CuS contains 33.3 % of sulphur, CuO contains 20.1% of oxygen and SO3 contains 40 % of sulphur. Show that the data illustrates the law of reciprocal proportions.
Solution:

Consider CuS
% of sulphur = 33.3
% of sulphur = 100 – 33.3 = 66.7
66.7 g of copper combines with 33.3 g of sulphur.
Hence 1 g of copper combines with 33.3 / 66.7= 0.5 g of sulphur.
Consider CuO
% of oxygen = 20.1
% of copper = 100 – 20.1 = 79.9
79.9 g of copper combines with 20.1 g of oxygen.
Hence 1 g of carbon combines with 20.1 / 79.9= 0.25 g of oxygen.
The ratio of different masses of sulphur and oxygen combining with fixed mass of copper is 0.5 : 0.25
i.e. 2 : 1. ………………….. (Ratio 1)
Consider SO3
% of sulphur = 40
% of oxygen = 100 – 40 = 60
40 g of sulphur combines with 60 g of oxygen.
In the next article, we shall study Gay-Lussac’s law of combining volumes.
Previous Topic: The Law of Multiple Proportions
Nex Topic: Gay-Lussac’s Law of Combining Volumes