Science > Chemistry > Introduction to Chemistry > Naming of Chemical Compounds
In this article, we shall study the method of naming of chemical compounds
In a chemical reaction, the molecular composition changes and it is represented by a chemical equation. In a chemical equation, the various substances involved as reactants or products are written in the form of symbols and formulae. These symbols and formulae are shorthand notations of the molecules involved as reactants or products.
Symbol of an Element:
A symbol is a representation of an atom of an element. These are unique 1,2, and 3 letter symbols. The first letter of an Element symbol is always a capital letter and remaining letters are written in lower case only. Many of the element symbols reflect the English name of the element such as Oxygen (O), hydrogen (H), carbon (C), nitrogen.(N), etc. These are single letter symbols. Examples of double letter symbols are aluminium (Al), Magnesium (Mg), calcium (Ca), etc.. Some symbols are derived from Latin names Aurum (gold) (Au), Argentum (silver), Stannum (tin) (Sn), Plumbum (lead) (Pb), etc.(Ag),
The formula of Element or Compound:
The formula is a representation of the actual number of atom or atoms of each element present in one molecule of the substance. The formula of element or compound is written by making use of the symbols of the respective elements. The formula for nitrogen is N2, that of oxygen is O2, that of carbon dioxide is CO2, etc.
Radicals or Ions:
A radical is a group of atoms of elements carrying a charge, e.g., chlorate [ClO3–].
Radicals or ions are formed by losing or gaining electrons. When an electron is gained the group of atoms acquire a negative charge and is called a negative radical or negative ion. When an electron is lost the group of atoms acquire a positive charge and is called a positive radical or positive ion. Depending upon the number of charges carried by radicals they are further classified as monovalent, bivalent (divalent), trivalent and tetravalent radicals or ions and so on.
Positive Radicals:
| Valency | Radical | Symbol | Ion |
| Monovalent | Hydrogen | H | H + |
| Sodium | Na | Na + | |
| Potassium | K | K + | |
| Mercurous or Mercury (I) | Hg | Hg + | |
| Cuprous or Copper (I) | Cu | Cu + | |
| Argenous or silver (I) | Ag | Ag + | |
| Divalent [2] | Zinc | Zn | Zn2+ |
| Magnesium | Mg | Mg2+ | |
| Calcium | Ca | Ca2+ | |
| Cupric or Copper (II) | Cu | Cu2+ | |
| Mercuric or Mercury (II) | Hg | Hg2+ | |
| Frrrous or Iron (II) | Fe | Fe2+ | |
| Barium | Ba | Ba2+ | |
| Stanous or Tin (II) | Sn | Sn2+ | |
| Plumbous or Lead (II) | Pb | Pb2+ | |
| Trivalent [3] | Aluminium | Al | Al3+ |
| Ferric or Iron (III) | Fe | Fe3+ | |
| Tetravalent [4] | Stanic or Tin (IV) | Sn | Sn4+ |
| Plumbic or Lead (IV) | Pb | Pb4+ |
Note:
In the case of the electropositive element showing variable valency. For lower valency, its name is written in form ending with ‘ous’ while with higher valency its name is written ending with ‘ic’. For example, iron shows two valencies 2 and 3. the iron showing lower valency 2 is named as ferrous and by the IUPAC system, it is named Iron (II). the iron showing higher valency r is named as ferric and by the IUPAC system, it is named Iron (III).
Negative Radicals:
| Valency | Radical | Symbol | Ion |
| Monovalent | Hydroxyl or hydroxide | OH | OH – |
| Fluoride | F | F – | |
| Chloride | Cl | Cl – | |
| Bromide | Br | Br – | |
| Iodide | I | I – | |
| Nitrite | NO2 | NO2 – | |
| Nitrate | NO3 | NO3– | |
| Hypochlorite | ClO | ClO – | |
| Chlorite | ClO2 | ClO2 – | |
| Chlorate | ClO3 | ClO3 – | |
| Perchlorate | ClO4 | ClO4 – | |
| Hypobromite | BrO | BrO – | |
| Bromite | BrO2 | BrO2 – | |
| Bromate | BrO3 | BrO3 – | |
| Perbromate | BrO4 | BrO4 – | |
| Hypoiodite | IO | IO – | |
| Iodite | IO2 | IO2 – | |
| Iodate | IO3 | IO3– | |
| Periodate | IO4 | IO4 – | |
| Acetate | CH3COO | CH3COO – | |
| Formate | HCOO | HCOO – | |
| Bisulphide | HS | HS – | |
| Bisulphite [Hydrogen sulphite] | HSO3 | HSO3 – | |
| Bisulphate [Hydrogen sulphate] | HSO4 | HSO4 – | |
| Bicarbonate [Hydrogen carbonate] | HCO3 | HCO3 – | |
| Cyanide | CN | CN – | |
| Cyanate | CNO | CNO – | |
| Thiocyanate | SCN | SCN – | |
| Permanganate | MnO4 | MnO4– | |
| Oxide | O | O2– | |
| Divalent [2] | |||
| Peroxide | O2 | O22- | |
| Carbonate | CO3 | CO32- | |
| Sulphide | S | S2- | |
| Sulphite | SO3 | SO32- | |
| Sulphate | SO4 | SO42- | |
| Thiosulphate | S2O3 | S2O32- | |
| Biphosphate [Hydrogen phosphate] | HPO4 | HPO42- | |
| Chromate | CrO4 | CrO42- | |
| Dichromate | Cr2O7 | Cr2O72- | |
| Oxalate | C2O4 | C2O42- | |
| Trivalent [3] | Phosphite | PO3 | PO33- |
| Phosphate | PO4 | PO43- |
Derivation of Formulae of Compound:
Steps Involved:
- First, write electropositive element (say A) in its radical form ( Ax+) followed by the electronegative element (say B) in its radical form ( Ay-). (Use tables for radicals).
- Below the element write their numeric charge without considering nature. ( x and y)
- Swap the numbers obtained in step 2 i.e. (y and x)
- Now the molecule should be electrically neutral. Hence the formula of the molecule is AyBx
Formula for ferric chloride:
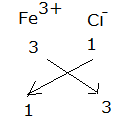
The formula for ferric chloride is FeCl3.
Formula for ferric oxide:
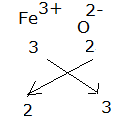
The formula for ferric oxide is Fe2.O3.
Formula for Aluminium hydroxide:

The formula for Aluminium hydroxide is Al(OH)3.
Formula for magnesium phosphite:

The formula for magnesium phosphate is Mg3(PO3)2.
Naming Binary Compounds:
General Prefixes Used in Naming:
| Number | Multiplier |
| 1 | mono- |
| 2 | di- |
| 3 | tri- |
| 4 | tetra- |
| 5 | penta- |
| 6 | hexa- |
| 7 | hepta- |
| 8 | octa- |
| 9 | nona- |
| 10 | deca- |
Steps Involved in Naming:
- Write the molecular formula. Identify radicals.
- Find the valencies of constituent groups. It is important for elements showing variable valency.
- Name of electropositive or less electronegative element/ group is written first. (due consideration to be given to elements showing variable valency). followed by name of the electronegative or more electronegative element or group.
| Compound | Name |
| NaCl | Sodium chloride |
| MgCl2 | Magnesium chloride |
| CaCl2 | Calcium chloride |
| BaO | Barium oxide |
| H2S | Hydrogen sulphide |
| FeCl3 | Ferric chloride or Iron (II) chloride |
| FeCl2 | Ferrous chloride or Iron (III) chloride |
| SnCl2 | Stannous chloride or Tin (II) chloride |
| SnCl4 | Stannic chloride or Tin (IV) chloride |
| CuCl | Cuprous chloride or Copper (I) chloride |
| CuCl2 | Cupric Chloride or Copper (II) chloride |
| CO | Carbon monoxide |
| CO2 | Carbon dioxide |
| SO2 | Sulphur dioxide |
| SO3 | Sulphur trioxide |
| CCl4 | Carbon tetrachloride |
| P2O5 | Phosphorous pentaoxide |
| UF6 | Uranium hexafluoride |
Naming of Acids:
Binary Acids:
They have general formula HA. The negative radical consists of a single non-metal. Use hydro as prefix and ic suffix.
| Binary Acid | Name |
| HF | Hydrofluoric acid |
| HCl | Hydrochloric acid |
| HBr | Hydrobromic acid |
| HI | Hydroiodic acid |
Binary Acid Name:
Oxyacids:
The negative radical consists of non-metal and oxygen. If the percentage of oxygen is less, then suffix ous is used. If the percentage of oxygen is more, then the suffix ic is used.
| Oxyacid | Name |
| H2SO3 | Sulphurous acid |
| H2SO4 | Sulphuric acid |
| HNO2 | Nitrous acid |
| HNO3 | Nitric acid |
| H3PO3 | Phosphorous acid |
| H3PO4 | Phosphoric acid |
If acid contains less number of oxygen than its corresponding ‘ous’ acid a prefix hypo is given to the negative radical. If acid contains more number of oxygen than its corresponding ‘ic’ acid a prefix per is given to the negative
| Acid | Name |
| HClO | Hypochlorous acid |
| HClO2 | Chlorous acid |
| HClO3 | Chloric acid |
| HClO4 | Perchloric acid |
Naming of Bases:
Base have general formula BOH. They contain hydroxyl (OH-) group. Metal is the electropositive radical and hydroxyl group is negative radical. The name of metal or group is written first followed by the word hydroxide.
| Base | Name |
| NaOH | Sodium hydroxide |
| KOH | Potassium hydroxide |
| Al(OH)3 | Aluminium hydroxide |
| Mg(OH)2 | Magnesium hydroxide |
| NH4OH | Ammonium hydroxide |
Naming of Salts:
Salt is assumed to be a product of neutralization reaction between acid and base. The positive radical of salt comes from the base while the negative radical comes from the acid. The name of salt is written with positive radical followed by negative radical.
In case of oxyacids, if the negative radical is derived from ic acid the suffix ate is used for naming the salt. if the negative radical is derived from ous acid the suffix ite is used for naming the salt.
Salts involving Binary acids:
| Salt | Name |
| NaCl | Sodium chloride |
| NH4Cl | Ammonium chloride |
| CH3COONa | Sodium acetate |
| MgCl2 | Magnesium chloride |
| KBr | Potassium bromide |
| KI | Potassium iodide |
Salts involving oxyacids:
| Salt | Corresponding Acid | Suffix | Name |
| ZnSO4 | Sulphuric acid ( H2SO4) | ate | Zinc sulphate |
| NaNO3 | Nitric acid (HNO3) | ate | Sodium nitrate |
| AlPO4 | Phosphoric acid (H3PO4) | ate | Aluminium phosphate |
| CaSO3 | Sulphurous acid ( H2SO3) | ite | Calcium sulphite |
| ZnNO2 | Nitrous acid ( HNO2) | ite | Zinc nitrite |
| Mg(PO)2 | Magnesium phosphate | ite | Magnesium phosphite |
In next article, we shall study next chapter: Laws of chemical combinations. In which we shall study dalton’s atomic theory, the law of conservation of mass, the law of definite proportion, the law of multiple proportions, the law of reciprocal proportions, Gay-Lussac’s law of combining volume.
Previous Topic: Methods of Separation of Mixtures
Next Topic: Laws of Chemical Combinations
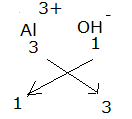
16 replies on “The Naming of Chemical Compounds”
Very good concept
Thanks
SAHI HAIN BHAI AAP KAHA SE HO
very good information
very very thank you
Brilliant concept v.v good and thanks
Thanks for the information.
It really answered my question.
Formula of Ferrous Chloride is given wrong.
Thank you for your support. Correction done.
Thanks a lot
Nice bro
Thanx
Waw what an explicit explanation
Nice explanation, it really helped me.
Thanks a lot.
Very comprehensive and detailed
It really helps me
Very very good imformation
Vv.good information thanks.
Very very very useful information 👍👍