Science > Chemistry > Molecule and Molecular Mass > Molecular Mass by Victor Meyer’s Method
In the last few articles, we have studied the molar volume method, Regnault’s method, Graham’s diffusion law method, and ideal gas equation method to determine molecular mass. In this article, we shall study Victor Meyer’s method to determine the molecular mass of a volatile substance.
Molecule:
When two or more atoms are firmly held together by a chemical bond, a molecule is formed. The molecule of an element may consist of one or more atoms of the same kind, while that of the chemical compound consists of different kinds of atoms.
The smallest particle of an element or compound which can exist in a free state and does not take part in a chemical reaction is called molecule.
Molecules are denoted by formula indicating the number of constituent elements in the compound. For example molecular formula for oxygen is O2. Thus one molecule oxygen consists of two atoms of oxygen
Molecular Mass or Molar Mass:
The molecular mass or molar mass of a substance is defined as the ratio of the mass of one molecule of a substance to 1/12 th of the mass of 6C12 isotope taken as 12000 units.
Gram Molecular Mass or Molar Mass:
The molecular mass expressed in grams is called gram molecular mass (GMM)
Method – IV (Molecular Mass by Victor Meyer’s Method):
Principle:
A known mass of volatile substance in Victor Meyer’s tube. As a result, the liquid changes into vapours, the vapours, in turn, displaces an equal volume of air which is collected over water. The volume of air collected is measured at the pressure and temperature of the laboratory. This volume is converted to S.T.P. volume and using gram molar volume concept molecular mass is calculated.
Procedure:
A round bottom flask filled with a liquid whose B.P. is 10 °C more than the volatile liquid acts as an outer glass jacket. A Victor Mayor’s tube with an outer tube acts as inner glass jacket. This outer tube is dipped in a trough fitted with water. Bottom of the Victor Mayor’s tube consists of Hg or asbestos pieces for cushioning.
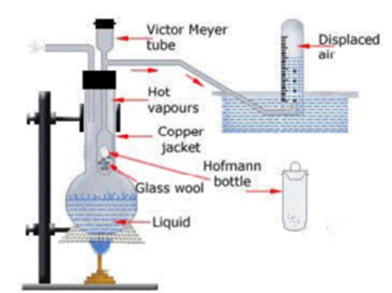
Outer glass jacket is heated due to which air inside expands and bubbles through the water in the trough. A small. glass tube is known as Hoffman’s bottle. with a stopper is cleaned, washed and dried and weighed. Volatile Liquid is taken in Hoffman’s bottle and weighed. A Eudiometer tube filled with water is placed over the tube connected to Victor Mayor apparatus.
Now Hoffman’s bottle is dropped in the Victor Mayor tube. Due to heat liquid in the bottle vapourises and blows off the stopper and displace air which corresponds to its own volume which is collected in a eudiometer tube by the downward displacement of water.
The water temperature and pressure are also noted. Eudiometer tube is taken into another trough filled with water for equalisation of pressure. The volume of air displaced is noted, which in fact correspond to the volume of the vapours.
Observations:
The weight of the Hoffman’s Bottle = W1 gm.
The weight of the Hoffman’s bottle + Liquid = W2 gm.
Weight of the volatile liquid = W2 – W1 = W gm.
Pressure = (p – f) mm of Hg.
Temperature = t °C = T K
Volume of vapours = V dm3.
Calculations:
Now the volume of vapours at S.T.P. from the above data is calculated using following formula.
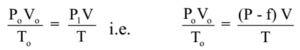
Where the volume of hydrogen at S.T.P. = V0 dm3
Pressure at S.T.P. = P0 mm of Hg = 760 m
Absolute temperature at S.T.P.= T K = 273 K
Now, the molecular weight can be calculated by following formula
Merits of Victor Mayer’s Method:
- The method is very simple to carry out. Weight.
- The sample required for the experiment is very small.
Demerits of Victor Mayer’s Method:
- This method is applicable to volatile liquid only.
- The method can not be used for the substance which undergoes thermal decomposition.
- The liquid in outer jacket is generally water, hence this method is suitable only for the liquids which have boiling point less than 100oC.
- Due to manual handling, there is the possibility of personal error.
- It is not applicable to volatile substances which are water soluble.
Numerical Problems:
Example – 01:
In the determination of molecular mass by Victor Meyer’s method 0.60 g of volatile substance expelled 123 ml of air measured over water at 20 °C and 757.4 mm pressure. Find the molecular mass of the substance if the aqueous tension at 20 °C is 17.4 mm.
Given: V = 123 ml = 0.123 dm³, t = 20 °C, T = 20 + 273 = 293 K, P = 757.4 mm of Hg , f = 17.4 mm of Hg, P – f = 757.4 – 17.4 = 740 mm of Hg, Po = 760 mm of Hg, To = 273 K
Solution:
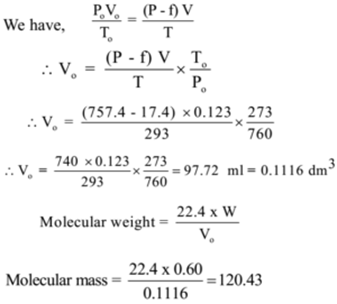
Ans: The molecular mass of the substance is 120.43
Method – V (Duma’s Method):
Procedure:
In this method, a known volume of vapour is heated to some higher temperature and its mass is noted. The calculations are the same as Victor Meyer’s method.
Method – VII (Hoffman’s Method):
Procedure:
Some substances are decomposed when they are evaporated at their boiling points. The substance is evaporated at a sufficiently low temperature at reduced pressure. Hoffman’s method is used for calculating the molecular mass of such substances.
A known mass of a substance is vapourised above a mercury column in a barometric tube and the volume of vapour formed is noted, Then the volume is calculated at STP, and calculation is done the same way as in Victor Meyer’s method.