Science > Physics > Electrostatics > Dielectrics
In this article, we shall study dielectric materials (dielectrics), their working and types.
Dielectrics are non-conducting substances. In these materials, free-moving electrons are not present. Thus there is no question of the movement of charge. When the dielectric material is kept in an external electric field, a dipole movement is introduced in the dielectric due to the stretching and reorientation of the molecules of the dielectric. Due to this molecular dipoles, a charge is created on the surface of the dielectric. Which produces an electric field that opposes the external electric field.
- Examples of solid dielectrics: Ceramics, glasses, plastics, rubber, mica asbestose.
- Examples of liquid dielectrics: Mineral oil, silicone oil, magnesia.
Types of Dielectrics:
Polar Dielectrics:
A polar molecule is one in which the centre of gravity of positive nuclei and revolving electrons do not coincide. Examples: HCl, H2O, N2O molecules.
Polar molecules have a permanent dipole moment. Thus they behave like a tiny electric dipole. In the absence of an external electric field, the tiny molecular electric dipoles are randomly arranged due to thermal agitation. Thus the net electric dipole moment of the polar dielectric is zero. In presence of an external electric field, these tiny molecular electric dipoles align themselves in the direction of the external electric field. Thus there is net dipole moment in the direction of the field.
Non-Polar Dielectrics:
A non-polar molecule is one in which the centre of gravity of positive nuclei and revolving electrons coincide. Examples: O2, H2, CO2, Polyethene, polystyrene.
Due to symmetry non-polar molecules do not have a permanent dipole moment. When non-polar molecules are subjected to an external electric field, the positive and negative charges in the molecules are displaced in the opposite direction. This displacement continues until the external force on the charges is balanced by restoring force due to the internal molecular field. Thus nonpolar molecules acquire induced dipole moment in the external electric field and are said to be polarised in the external electric field. The induced electric moments of different molecules add up and give rise to the net dipole moment.
Polarization:
When polar or a non-polar dielectric are kept in the external electric field, their molecules acquire induced dipole moment and the dielectric is said to be polarized in the external electric field. The phenomenon is known as polarization. Polarization is defined as dipole moment per unit volume and is given by

This relation is true for linear isotropic dielectrics.
Linear isotropic dielectrics are those dielectrics in which induced dipole moment is induced in the same direction of the external field and is proportional to the field strength.
Behaviour of Dielectric Slab Which is Subjected to External Electric Field:
Consider a thin slab of the dielectric of permittivity placed in an external uniform electric field. Irrespective of the nature of their molecules (polar or non-polar) the dielectric gets polarised. Due to polarization molecules are oriented such that the negative charges are on the left side and positive charges on the right side. The net electric charge on the dielectric is zero. The charges so obtained on the surface of the dielectric slab are called polarization charges.
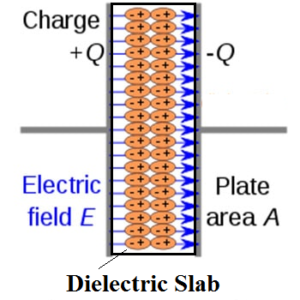
Thus polarized dielectric is equivalent to two charged surfaces with polarization charges. These charges oppose the external electric field and thereby weaken the original field within the dielectric.
Polarization on the dielectric slab can be defined as the amount of induced surface charge per unit area (area right angle to the external electric field) of the surface.

Where, P = Polarization
qP = Polarization charges
σP = Charge density of polarization charges
A = Area of cross-section of dielectric
P is a vector quantity and is directed from negative induced charges to positive induced charges.
Proof:
Polarization is defined as dipole moment per unit volume

Where,Q = nq = Net charge on all dipoles
n = number of molecular dipoles
q = charge of each dipole
l = length
A = area of cross-section of dipole
Thus polarization can be also defined as the amount of induced surface charge per unit area of the surface.
Previous Topic: Mechanical Force per Unit Area of Charged Conductor
Next Topic: Concept of Capacity of a Conductor