Science > Physics > Introduction to Measurements > Concept Application – 01
Introduction to Measurements
LIST OF SUB-TOPICS:
Fill in the Blanks:
Fill in the blanks with appropriate words.
- There are ……. fundamental and ……. supplementary units in S.I. system.
- The measurement of a physical quantity is the product of the numerical value and ……….. of that quantity.
- The S.I. unit of acceleration is …….
- The S.I. unit of force is …….
- The S.I. unit of energy is ……
- The S.I. unit of linear momentum is …….
- S.I. unit of angular momentum is …….
- The c.g.s. unit of acceleration is …….
- The c.g.s. unit of force is …….
- The c.g.s. unit of energy is ……..
- The c.g.s. unit of linear momentum is ……
- Metre per second is the unit of ………… and ……….
- The supplementary units of S.I. system are ………. And ………….
- Candela is a ……. unit.
- Ampere is S.I. unit of …….
- Angstrom is a unit for …….
- S. I. unit of luminous intensity is ………..
- 1 angstrom is equal to ……. cm
- 1 astronomical unit equals to …………. cm.
- S.I. unit of plane angle is ………..
- S.I. unit of solid angle is ………..
Answers:
| 1) seven, two | 2) unit |
| 3) m s-1 | 4) newton (N) |
| 5) joule (J) | 6) kg ms-1 |
| 7) kg m2 s-1 | 8) cm s-1 |
| 9) dyne | 10) erg |
| 11) g cm2 s-1 | 12) speed, velocity |
| 13) radian, steradian | 14) Luminous intensity |
| 15) Electric current | 16) Length |
| 17) candela (cd) | 18) 108 |
| 19) 1.5 x 1013 | 20) radian |
| 21) steradian |
True or False:
State whether the following statements are true or false.
- The magnitude of a quantity change with change in the unit of system.
- If numerical value of quantity is x in c.g.s. system and y in m.k.s. system then x must be less than y
- Parsec is largest practical unit of length.
- The S.I. unit of amount of substance is mole.
- The S.I. unit of acceleration is m s-1.
- The S.I. unit of surface tension is N m-1.
- Newton is a fundamental unit.
- Steradian is a supplementary unit.
- Light year is unit of time.
- Shake is a unit of time.
- The c.g.s. unit of force is dyne.
- 1 angstrom is equal to 10-10 m.
- Solar day is a measure of time.
- The units of torque and work are same.
- The unit of magnetic energy, electrical energy, and mechanical energy is same.
Answers:
| 1) True | 2) False | 3) True |
| 4) True | 5) True | 6) True |
| 7) False | 8) True | 9) False |
| 10) True | 11) True | 12) True |
| 13) True | 14) True | 15) True |
Very Short Answer:
Q1. What are physical quantities?
Physical quantities are those quantities that are measurable. Example: Length, mass, time, force, momentum, density, etc.
Q2. What are fundamental quantities? Give Examples.
Fundamental quantities are those quantities that do not depend on other quantities for their measurements. For Example mass, length, time, etc. are fundamental quantities.
Q3. What are derived quantities? Give examples.
Derived quantities are those quantities that depend on two or more other quantities for their measurements. For example, density, acceleration, velocity, force, momentum, pressure, etc. are derived quantities
Q4. What are fundamental units?
Fundamental quantities are those quantities that do not depend on other quantities for their measurements. The units of fundamental quantities are called fundamental units. For Example mass, length, time, etc. are fundamental quantities, while, their units metre, kilogram, second, etc. are fundamental units.
Q5. What are derived units?
Derived quantities are those quantities that depend on two or more other quantities for their measurements. The units of derived quantities are called derived units. For example, density, acceleration, velocity, force, momentum, pressure, etc. are derived quantities.
Q6. What is a unit of physical quantity?
A unit is a selected magnitude of a physical variable in terms of which other magnitudes of the same variable can be expressed. Measurement without unit has no meaning.
Q7. Mention various systems of fundamental units.
A system of units is a collection of units in which certain units are chosen as fundamental and all others are derived from them. The following are some systems of units that are in common use.
- S.I. System of units
- c.g.s. system of units
- m.k.s. system of units
- f.p.s. system of units
Q8. Mention fundamental units in c.g.s. system.
The unit of length is centimetre (cm). The unit of mass is gram (g). The unit of time is second (s).
Q9. Mention fundamental units in m.k.s. system.
The unit of length is the metre (m). The unit of mass is the kilogram (kg). The unit of time is second (s).
Q10. Mention fundamental units in f.p.s. system.
The unit of length is the foot (ft). The unit of mass is a pound (Lb). The unit of time is second (s). This system is no more in use.
Q11. Gine convenient units to be used for measuring the following quantities.
| Sr. No. | Physical Quantity | Convenient Unit |
| 1 | Distance between two cities | kilometre (km) |
| 2 | Length of your classroom | metre (m) |
| 3 | Height of Eifel Tower | metre (m) |
| 4 | Thickness of single hair | micrometer ( μ m) |
| 5 | Diameter of a teacup | centimetre (cm) |
Q12. If Impulse = Force × Time and the S.I. unit of force is newton (N), then S.I. unit of impulse is newton second ………….
Impulse = Force × Time
∴ S.I. Unit of Impulse = S.I. Unit of Force × S.I. Unit of Time
∴ S.I. Unit of Impulse = newton (N) × second (s)
∴ S.I. Unit of Impulse = newton second (Ns)
No need to write the above explanation. It is only for reference.
If Impulse = Force × Time and the S.I. unit of force is newton (N), then S.I. unit of impulse is newton second (Ns).
Q13. Define the following.
- Kilogram: One kilogram is the mass of a cylinder made up of platinum-iridium alloy kept at the International Beuro of Weights and Measure.
- Metre: One metre is 1,650, 763.73 times the wavelength of orange light emitte by a krypton atom at normal pressure.
- Second: 1 second is a time duration of 9,192,631,770 periods of the radiation corresponding to the transition between two hyperfine levels of the ground state of the Cesium-133 atom.
Q 14. The number of waves of orange light emitted by krypton in 1 m is ……..
The number of waves of orange light emitted by krypton in 1 m is 1,650, 763.73.
Q15. The time taken by light emitted by the cesium-133 atom to complete 9,192, 631, 770 vibrations is ………. s.
The time taken by light emitted by the cesium-133 atom to complete 9,192, 631, 770 vibrations is 1 s.
Q16. In a given system of units, the ratio of the unit of volume to that of the area gives a unit of ………..
Required ratio = Unit of volume/Unit of area = m3/m2 = m = unit of length
In a given system of units, the ratio of the unit of volume to that of the area gives a unit of length.
Q17. In a new system of units to be introduced, if the unit of length is 2 m and 2 s is considered as unit time, then unit speed is equal to ………..
Speed = Distance / Time = 2 m/ 2s = 1 m s-1
In a new system of units to be introduced, if the unit of length is 2 m and 2 s is considered as unit time, then unit speed is equal to 1 m s-1.
Short Answers:
Q1. What are the different types of measurements?
Depending on the method, measurements are classified into two types: a) Direct measurement and b) Indirect measurement
- Direct Measurement: When measurements are taken directly using tools, instruments, or other calibrated measuring devices, they are called direct measurements e.g. Measurement of the length of a table by metre scale.
- Indirect Measurement: When the measurement must be done through a formula or other calculations, the measurement is called indirect measurement e.g. Measurement of the radius of the Earth.
Q2. What are the characteristics of a standard unit?
The characteristics of the standard are as follows:
- The standard should be easily available.
- The standard should be non-destructible.
- The standard should not change with the time.
- The standard should not change with the place.
- The standard should be easily reproducible
Q3. What are the characteristics of a unit of a physical quantity?
The characteristics of a unit of a physical quantity are as follows:
- It should be well defined without any doubt or ambiguity.
- It should be of suitable size. i.e. neither too long nor too small in comparison with quantity to be measured.
- It should be easily available.It should be non-destructible.
- It should not change with time.
- It should not change with the place.
- It should be easily reproducible.
Q4. List the fundamental and supplementary quantities in S.I. system along with their units.
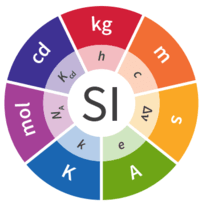
Fundamental Quantities and Their Units:
| Fundamental Quantity | S.I. Unit | Symbol | |
| 1 | Length | Metre | m |
| 2 | Mass | Kilogram | kg |
| 3 | Time | Second | s |
| 4 | Temperature | Kelvin | K |
| 5 | Electric current | Ampere | A |
| 6 | Luminous intensity | Candela | cd |
| 7 | Amount of substance | mple | mol |
Besides these seven basic units, there are two supplementary units. S.I. unit for the plane angle is radian (rad) and that of solid angle is steradian (sd).
Supplementary Quantities and Their Units:
| Quantity | S.I. Unit | Symbol | |
| 1 | Plane angle | radian | rad |
| 2 | Solid angle | steradian | sr |
Q5. Explain the steps involved in finding a derived unit of a physical quantity.
- Step -1: Write the formula for the quantity whose unit is to be derived.
- Step -2: Substitute units of all the quantities in one system of units in their fundamental or standard form.
- Step -3: Simplify and obtain derive unit of the quantity.
Example: To find the unit of velocity.
Velocity is a derived quantity. Hence its unit is a derived unit.
The velocity is given by, velocity = displacement/time
S.I. unit of velocity = S.I. unit of displacement/ S.I. unit of time = m/s
Thus S.I. unit of velocity is m/s
Essay Type Answers:
Q1. Explain the need for measurement?
Measurement is that operation by which we compare a physical quantity with a unit chosen for that quantity. In science and engineering, we perform experiments. During experiments, we have to take readings. Thus all these experiments require some measurements to be made. During the production of mechanical products, we have to measure the parts so as to find whether the part is made as per the specifications. Thus measurements are necessary for production and quality control.
Q2. Explain the criteria of the selection of a unit for the measurement.
The selection of a unit depends on the magnitude of a quantity under consideration. For e.g. when we are measuring the diameter of a rod we should use millimetre as a unit. When we are measuring the height of a tower we should use the metre as a unit. When we are measuring the distance between the two cities we should use kilometre as a unit. When we are measuring the distance between the two stars we should use light-years as a unit. This clearly indicates that when the magnitude of the measurement increases, then the unit used for the measurement should be larger. The unit should be neither too small nor too big in comparison with the physical quantity to be measured.
The accuracy of measurement also influences the selection of the unit. In the case of construction of a room where accuracy is not a major criteria metre or foot are used as units. But when constructing a rocket, accuracy is important hence millimetre or micrometre may be the unit. Thus when the accuracy is important then the unit used for the measurement should be smaller.
Q3. Explain the need for standards and system of units in measurement.
When humans became civilised, started cultivating and living in communities they realised that one cannot do everything and they need to be interdependent. This paved the way for trade and then probably a need of a measure was felt. The recorded history shows ample evidence that the different parts of the human body were used as a point of reference while making measurements. The units based on parts of human body are arbitrary and inaccurate. The results of the measurements vary from person to person because size of the unit is different for different person. This created problems in trade between different countries and also in the day to day transactions. In order to overcome the limitations of body parts as units, and to bring about uniformity in the measurement system, the need for exact measurement was felt. For this, a standard of measurements had to be developed which is acceptable to everybody and to make our judgement more reliable and accurate. Thus there should be uniformity in measurement. For the sake of uniformity we need a common set of units of measurement, which are called standard units. Nowadays S.I. units in science and technology are almost accepted universally.
Q4. Give guidelines for writing a unit of a physical quantity.
- All units and their symbols should be written in small case letters e.g. centimetres (cm), metre (m), kilogram per metre cube ( kg m-3).
- The units named after scientists are not written with a capital initial letter but its symbol is written in capital letter. Thus the unit of force is written as ‘newton’ or’ N’ and not as ‘Newton’. Similarly unit of work and energy is joule (J), S.I. unit of electric current is ampere (A). The S.I. nit of pressure is pascal (Pa) and that of temperature is kelvin (K).
- No full stop should be placed after the symbol.
- The denominators in a compound unit should be written with negative powers. Thus an index notation should be used to write a derived unit. for example unit of velocity should be written as ms-1 instead of m/s. The unit of density is kilogram per metre cube ( kg m-3 and not kg/m3)
- No plural form of a unit or its symbol should be used. example 5 newtons should be written as 5 N and not as 5 Ns.
- A compound unit obtained from units of two or more physical quantities is written either by putting a dot or leaving a space between symbols of two units. Example unit of torque is newton metre is written as Nm ot N.m. Unit of impulse is newton second is written as N s or N.s.
- Some space should be maintained between the number and its unit.
Q5. What are the advantages of the S.I. system of units?
- Units are simple to express
- This system uses only one unit for one physical quantity. Hence it is a rational system of units.
- Units of many physical quantities are related to each other through simple and elementary relationships For example 1 ampere = 1 volt / 1 ohm.It is a metric system of units.
- There is a decimal relationship between the units of the same quantity and hence it is possible to express any small or large quantity as a power of 10. i.e. inter-conversion is very easy. For e.g. 1kg = 1000 gm = 10³ gm
- The physical quantities can be expressed in terms of suitable prefixes.
- A joule is a unit of all forms of energy and it is a unit of work. Hence it forms a link between mechanical and electrical units. Hence S.I. the system is a rational system because it uses only one unit for one physical quantity.
- This system forms a logical and interconnected framework for all measurements in science, technology, and commerce.
- All derived units can be obtained by dividing and multiplying the basic and supplementary units and no numerical factors are introduced as in another system of units. Hence S.I. system of units is a coherent system. Hence S.I. system of units is used worldwide.
Q6. what are the responsibilities of National Physical Laboratory (NPL)?
- It is the responsibility of the NPL to calibrate the measurement standards in these laboratories at different levels.
- The weights and balances used in local markets and other areas are expected to be certified by the Department of Weights and Measures of the local government.
- To strengthen and advance physics-based research and development for the overall development of science and technology in the country.
- To establish, maintain and improve continuously by research, for the benefit of the nation,
- To identify and conduct after due consideration, research in areas of physics which are most appropriate to the needs of the nation and for the advancement of the field
- To assist industries, national and other agencies in their developmental tasks by precision measurements, calibration, development of devices, processes, and other allied problems related to physics.
- To keep itself informed of and study critically the status of physics.
Related Topics:
- 1.2.1 Introduction to Measurements
- 1.2.2 Meassurement of Length, Area and Volume
- 1.2.3 Measurement of Mass, Weight, and Density
- 1.2.4 Measurement of Time
- 1.2.5 Dimensional Analysis
- 1.2.6 Error Analysis