Science > Chemistry > Third Row Elements > Ionization Enthalpy of Third Row Elements
In this article, we shall study trend in the ionization enthalpy of third-row elements.
Ionization Enthalpy:
The minimum energy required to remove the most loosely attached electron from the outermost shell of a neutral gaseous, isolated atom of an element in its ground state to produce gaseous cation is known as ionization enthalpy or ionization potential of that element. Ionization enthalpy is expressed in terms of kJ per mole
M(g) + I. P. → M(g)+ + e–
Where M is the third-row element
Factors Affecting the Ionization Enthalpy:
- The size (atomic radius) of an atom i.e. the distance of the outermost electron from the nucleus.
- The charge on the nucleus or the nuclear charge i.e. protons present in the nucleus.
- The screening effect.
- The type or the geometry of the subshell in which the electron is present.
- For the third row elements, the screening effect for all the elements is almost the same.
Trends in the Ionization Enthalpy Across the Period:
With some minor exceptions, the trend in ionization enthalpy of third-row elements is that as we move from left to the right i.e. from Sodium to Argon along period, ionization enthalpy of these elements goes on increasing steadily.
Its values for the third-row elements are given below.
| Element | Na | Mg | Al | Si | P | S | CI | Ar |
| Atomic Number (Z) | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 |
| First Ionization Enthalpy in kJ/mole | 496 | 737 | 577 | 786 | 1012 | 999 | 1252 | 1520 |
The irregularity in the trend can be observed from the following graph.
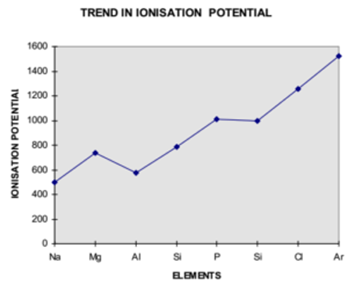
The ionization enthalpy of magnesium is more than aluminium. The ionization enthalpy of phosphorous is more than sulphur. The last element argon has the highest ionization enthalpy. Magnesium, phosphorous and argon have more ionization enthalpy than expected due to the extra stability of their exactly half filled and completely filled orbitals.
Scientific Reasons:
Ionization enthalpy of third-row elements goes on increasing steadily along the period.
Ionization enthalpy of an element depends upon i) atomic size ii) nuclear charge iii) screening effect.
If the element has smaller atomic size, greater nuclear charge and less dense inter electronic cloud, then it has greater ionization enthalpy. If the element has bigger atomic size, low nuclear charge and denser electronic cloud then it has low ionization potential.
Along the third period i.e. from Na to Ar, as atomic number increases, the nuclear charge goes on increasing, atomic size goes on decreasing, and the number of valence electron goes on increasing. The attractive force on the valence electron of an atom increases. Hence Ionization enthalpy of the third-row elements goes on increasing steadily along the period.
The ionisation enthalpy of magnesium is more than aluminium.
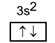
The atomic number of magnesium is 12. Its electronic configuration is 2, 8, 2. Its detailed configuration is 1s22s2 2p63s2. The box diagram for final orbit configuration for magnesium is as below. We can see that Mg has completely filled ‘3s’ orbitals.
The atomic number of aluminium is 13. Its electronic configuration is 2, 8, 3. Its detailed configuration is 1s22s2 p63s23p1. The box diagram for final orbit configuration for aluminium is as below. We can see that aluminium has partially filled ‘3p’ orbitals.
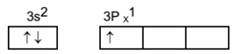
It has been observed that the extra stability is associated with vacant, half filled and completely filled orbitals. Magnesium has thus extra stable electronic state due to completely filled ‘3s’ orbital. It is difficult to remove an electron from pair due to extra stability. While aluminium has no such extra stable state. There is one unpaired electron in the 3p subshell of aluminium.
s orbitals are closer to the nucleus than p orbitals. So energy required to remove the electron from s orbital is always more than to remove it from p orbital. In the case of magnesium, the electron is to be removed from s orbital while in case of aluminium it is to be removed from p orbitals. Thus more energy will be required to remove valence electron from magnesium atom than that of aluminium. So ionization enthalpy of magnesium is greater than that of the aluminium.
The ionisation enthalpy of phosphorous is more than sulphur.
The atomic number of Phosphorous is 15. Its electronic configuration is 2, 8, 5. Its detail configuration is 1s2, 2s2 2p6, 3s2 3p3. The box diagram for final orbit configuration for phosphorous is as below. We can see that the p orbitals are exactly half filled.

The atomic number of Sulphur is 16. Its electronic configuration is 2, 8, 6.Its detail configuration is 1s2, 2s2 2p6, 3s2 3p4. The box diagram for final orbit configuration for sulphur is as below. We can see that sulphur has partially filled ‘3p’ orbitals.

It has been observed that the extra stability is associated with vacant, half-filled and completely filled orbitals. Phosphorous has thus extra stable electronic state due to exactly half filled ‘3p’ orbital. Sulphur has no such extra stable state as there are two unpaired electrons in 3p subshell. In sulphur, removing one electron from 3p orbital, it becomes half-filled and attains the stable state. Thus sulphur tries to lose electron fast. Thus more energy will be required to remove valence electron of phosphorous than that of sulphur. So ionization potential of phosphorous is greater than that of the sulphur.
Argon has the highest ionization potential.
The atomic number of argon is 18. Its electronic configuration is 2, 8, 8. Its detail configuration is 1s2, 2s2 2p6, 3s2 3p6. The box diagram for final orbit configuration for argon is as below. We can see that argon has completely filled ‘3s’ and ‘3p’ orbitals.

It has been observed that the extra stability is associated with empty, half-filled and completely filled orbitals. Argon the as most stable electronic configuration. It has a complete octet of electrons in the outer most shell. All electrons are paired. It is very difficult to remove valence electrons. Hence much more energy is required to remove an electron from such a stable configuration. So Argon has the highest ionization potential.
Concept of Higher Ionization Potentials:

Aluminium rarely forms the tri-positive cation.
Aluminium has atomic number 13. Its electronic configuration is 1s22s2 p63s23p1. To form a tri-positive ion of aluminium all the three electrons of the outermost shell are to be removed.
Formation of mono-positive ion i.e. cation involves the removal of an electron from a neutral atom. Hence the first ionization potential is always low. Formation of di-positive ions involves removal of an electron from mono-positive cation while the formation of tri-positive ion involves removal of an electron from di-positive cation. Due to the positive charge on the mono-positive and dipositive cations, the outgoing electron has to overcome larger attractive force.
Hence more energy is required to remove the second electron and still more energy is required to remove the third electron. Hence Aluminium rarely forms tri-positive ions.