Science > Chemistry > Third Row Elements > Introduction
The elements are arranged in the order of increasing atomic numbers, in such a way that elements with similar properties fall in the same vertical column of the periodic table. There are eighteen vertical columns known as groups and seven horizontal rows are known as periods. A group of eight elements namely, sodium (Na), magnesium (Mg), aluminium (Al), silicon (Si), phosphorous (P), sulphur (S), chlorine (Cl) and argon (Ar) belongs to the third period of the periodic table are called as third-row elements.
The properties of each element are characteristics of the group to which they belong. Each one of these elements can, therefore, be considered as the representative of the whole group to which it belongs. Hence these elements are known as typical or representative elements.
The properties of these elements gradually change from metallic or basic to non-metallic or acidic character across the third period. Sodium, magnesium and aluminium are metals. They have a metallic lustre, can conduct heat and electricity. They are malleable and ductile. Sodium is a very soft metal. Silicon is a hard solid and is metalloid. Phosphorous is a yellow waxy solid. It is non-metal. Sulphur is a yellow coloured solid. It is a non-metal. Chlorine and argon are gases at room temperature. They are non-metals.
Position of the Third Row Elements in the Periodic table:
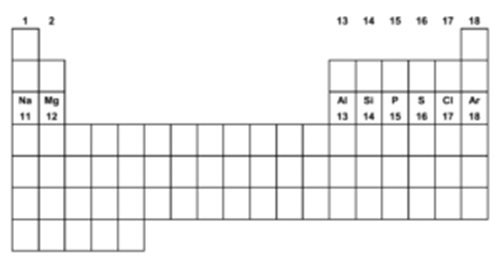
In the modern periodic table, third row elements have been placed in 1, 2, 13, 14, 15, 16, 17 and 18 groups. Except for Sodium and Argon, the third-row elements are the second member of their group. Sodium and Argon are the third members of their groups 1 and 18 respectively
For sodium and magnesium, the last electron to be configured enter into ‘s’ orbitals. Hence sodium and magnesium are s block elements. For all the other third row elements the last electron to be configured enter into ‘ ‘p’ orbitals hence they are p block elements.
There are three orbits and valence electrons are present in the third main energy level. All these elements have vacant 3d orbitals. These elements are capable of transferring their valence electrons to these vacant d orbitals. Thus they are capable of expanding their octet which is not possible in the second-row elements
Electronic Configuration of Third Row Elements:
Electronic configuration of third row elements of the third row is as follows
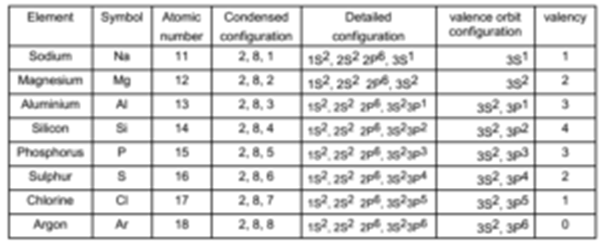
It can be seen that Sodium (Z = 11) has a single electron in its 3 s valence orbital. With the increase in atomic number, in magnesium, aluminium, silicon, phosphorous, and chlorine, the electrons successively occupy 3s and 3p valence orbitals until another closed-shell configuration 1s22s2 2p63s23p6 is reached at argon (Z = 18).
One reply on “Introduction to the Third Row Elements”
Good