Science > Physics > Surface Tension > Angle of Contact
When water is taken in a glass vessel, the free surface of the water near the walls is curved concave upward. If mercury is taken in a glass vessel, the free surface of mercury near the walls is convex upwards.
When the liquid is in contact with solid, the angle between the solid surface and the tangent to the free surface of the liquid at the point of contact, measured from inside the liquid is called the angle of contact.
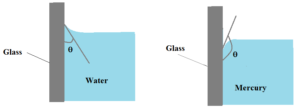
When the liquid surface is curved concave upwards, the angle of contact is acute and when the liquid surface is curved convex upwards, the angle of contact is obtuse.
Characteristics of the Angle of Contact:
- The angle of contact is constant for a given liquid-solid pair.
- When the angle of contact between the liquid and a solid surface is small (acute), the liquid is said to wet the surface. Thus water wets glass.
- If the angle of contact is large the surface is not wetted. Mercury does not wet glass.
- If there are impurities in liquid, then they alter the values of the angle of contact.
- The angle of contact decreases with an increase in temperature.
- For a liquid which completely wets the solid, the angle of contact is equal to zero.
Notes:
- The smearing of glycerine on a glass increases the angle of contact of water with glass from acute to obtuse, hence a glass window is smeared with glycerine.
- Smaller the angle of contact, better is the detergent.
- Teflon coating is done on the surface of the non-stick pan because Teflon increases the angle of contact from acute to obtuse.
The Shape of a Liquid Drop on Solid Surface:
When a small quantity of liquid is poured on a plane solid surface, a force of surface tension acts along a surface separating the two media. There is a formation of the liquid drop.
When the drop is in equilibrium, its shape depends on the forces of interface media. Thus there is surface tension along a surface between a) liquid and air b) solid and air and c) liquid and solid.
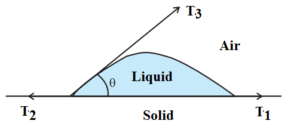
Let, θ = the angle of contact of given solid-liquid pair.
T1 = Surface tension at the liquid-solid interface
T2 = Surface tension at the air-solid interface
T3 = Surface tension at the air-liquid interface
For equilibrium of the drop
T2 = T1 + T3 cos θ

Case -1:
If T2 > T1, and T2 -T1 < T3, then cos θ is positive and the angle of contact is acute. e.g. kerosene on the glass surface

Case – 2:
If T2 < T1, and T1 -T2 < T3, then cos θ is negative and the angle of contact is obtuse. e.g. mercury on the glass surface Mercury does not wet the glass and thus it forms a drop on the glass.

Case – 3:
If T2 -T1 = T3, then cos θ is 1 and the angle of contact is zero. For pure water, cos θ is almost 1 i.e. water wets the glass and thus water spreads on the glass.
Case – 4:
If T2 -T1 > T3, then cos θ > 1 which is not possible. Thus liquid will spread over the solid and drop shall not be formed.
Notes:
For the small drop, gravitational potential energy is very small and hence can be neglected. Hence for stability, the surface tension of the drop should be least i.e. the surface area should be least. It can be achieved by forming a spherical drop because the surface area of a sphere is least for the given volume.
For the large drop, gravitational potential energy dominates to make the potential energy minimum. It does so by bringing the centre of gravity as low as possible, due to which the drop flattens.

The Formation of Concave Surface of Liquid (Explanation of Acute Angle of Contact):
When impure water or kerosene is taken in a glass vessel, it is found that the surface near the walls is curved concave upwards.

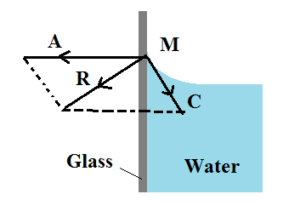
Consider a molecule of water M on the free surface very close to the wall of the glass vessel. The force of cohesion C due to other water molecules is as shown in the figure. In addition to this, a force of adhesion A acts due to the glass molecule as shown in the figure. The net adhesive force between water molecules and air molecules is negligible. The gravitational force on the molecule is also negligible.
The magnitude of A is greater than the magnitude of C and resultant of the two molecular forces of attraction R is directed towards the glass or outside the liquid. Hence the molecule A is attracted towards the walls of the glass vessel.
The free surface of water adjusts itself at right angles to the resultant R. Therefore molecules like M creep upward on the solid surface. Thus the water surface is curved concave upwards and the angle of contact is acute.
The Formation of Convex Surface of Liquid (Explanation of Obtuse Angle of Contact):
When mercury is taken in a glass vessel, it is found that the surface near the walls is curved convex upwards.

Consider a molecule of mercury M on the free surface very close to the wall of the glass vessel. The force of cohesion C due to other mercury molecules is as shown in the figure. In addition to this, a force of adhesion A acts due to the glass molecule as shown in the figure. The net adhesive force between mercury molecules and air molecules is negligible. The gravitational force on the molecule is also negligible.
The magnitude of A is very less than the magnitude of C and resultant of the two molecular forces of attraction R is directed inside the liquid. Hence the molecule A is attracted towards other molecules of mercury.
The free surface adjusts itself at right angles to the resultant R. The molecule A creeps downwards on the glass surface. Thus the surface of the mercury in the glass is curved convex upwards and the angle of contact is obtuse.
The Formation of Flat Surface of Liquid:
When pure is taken in a glass vessel, it is found that the surface of the water is perfectly flat.

Consider a molecule of water M on the free surface very close to the wall of the glass vessel. The force of cohesion C due to other water molecules is negligible. The force of adhesion A acts due to the glass molecule as shown in the figure. The gravitational force on the molecule is also negligible.
The magnitude of A is large and resultant of the two molecular forces of attraction R is along the direction of the adhesive force. Hence the molecule A is attracted towards glass molecules.
The free surface adjusts itself at right angles to the wall. Thus the surface of the pure water is perfectly flat.
Mercury Drops Coalesce Together When Brought Together:
The attractive force between the two molecules of the same substance is called as a cohesive force and the attractive force between the two molecules of the different substance is called an adhesive force.
In the case of mercury, the cohesive force between its molecules is very strong compared to negligible adhesive force between any surface and mercury molecules. The net adhesive force between mercury molecules and air molecules is negligible. The gravitational force on the molecule is also negligible. Thus there is a strong attraction between mercury molecules and almost no attractive force between surface and mercury molecules. Hence mercury drops coalesce together when brought together.
Water on a clean glass surface tends to spread out, while mercury on the same surface tends to form a drop.
The shape of the drop depends on the angle of contact of the liquid with glass. In the case of a glass water interface, the angle of contact is acute. Thus to achieve this angle water spreads on the glass. In the case of a glass mercury interface, the angle of contact is obtuse. Thus to achieve this angle mercury forms a drop as shown.
Previous Topic: Problems on Change in Surface Energy
Next Topic: Laplace’s Law of Spherical Membrane
3 replies on “Angle of Contact”
Much understandable and doesn’t make confusion I like it
This is an enough & standard notes about angles of contact.
Note: google ads should be stoped.
Very nice and sufficient explanation for contact angle