Science > Physics > Surface Tension > Surface Tension
In this article, we shall study the concept of the surface tension, its characteristics and the factors affecting it.
Intermolecular Forces:
Between any two molecules, there exists a force of attraction. This force is called the intermolecular force. These intermolecular forces of attraction are of two types. a) Cohesive force and b) Adhesive force.
Cohesive Force and Cohesion:
The attractive force between the two molecules of the same substance is called as a cohesive force and the attraction itself is called cohesion. e.g. attraction between water and water molecules.
Adhesive Force and Adhesion:
The attractive force between the two molecules of the different substance is called an adhesive force and the attraction itself is called adhesion. e.g. attraction between water and glass molecules.
Why does water wets glass?
The attractive force between the two molecules of the same substance is called as a cohesive force and the attractive force between the two molecules of the different substance is called an adhesive force. In case of water, the cohesive force between water molecules is very less compared to the adhesive force between glass and water molecules. Thus there is a strong attraction between water and glass molecules. Hence water wets glass.
Why does mercury not wet glass?
The attractive force between the two molecules of the same substance is called as a cohesive force and the attractive force between the two molecules of the different substance is called an adhesive force. In case of mercury, the cohesive force between its molecules is very strong compared to negligible adhesive force between glass and mercury molecules. Thus there is a strong attraction between mercury molecules and almost no attractive force between glass and mercury molecules. Hence mercury does not wet glass.
Range of molecular attraction (r):
The maximum distance between two molecules up to which the intermolecular forces are effective is called the range of molecular attraction. It is of order 10-9 m.
Sphere of Molecular Influence:
A sphere drawn by taking the radius equal to the range of molecular attraction and centre as the centre of the molecule is called sphere of molecular influence.
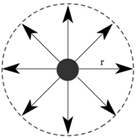
Concept of Surface Tension:
Explanation on the Basis of Molecular Theory:
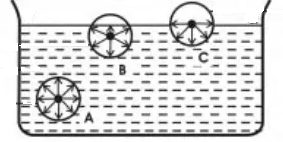
Consider three molecules A, B and C in a liquid, such that molecule A is well inside the liquid, molecule B is close to the free surface and the molecule C is on the free surface.
The sphere of influence of the molecule A is completely inside the liquid, and hence it will be acted upon by equal forces in all directions and these forces will balance one another and the net force acting on it is zero.
For molecule B, a part of the upper half of the sphere of influence is in the air, which contains air molecules. Air molecules exert very negligible adhesive forces on molecule B. Therefore, the cohesive forces due to molecules in the liquid remain unbalanced and thus a net force in downward direction acts on the molecule.
For the molecule C, the upper half of the sphere of influence is completely in the air. Due to this, the force of attraction due to the molecules inside the lower half of the sphere will remain unbalanced. This molecule experiences the maximum possible unbalanced force in the downward direction.
Thus the molecules on the surface and in a surface film of thickness equal to the range of molecular attraction of the liquid molecule experience a net force in the downward direction. The magnitude of the force depends upon the distance of the molecule from the free surface. The behaviour of this film is different from that of the rest of the liquid. It is called the surface film. This film behaves like a film which is under tension. This phenomenon is known as surface tension.
If any molecule is brought to the surface from the liquid, the work is to be done against this net downward force. This work increases the potential energy of the surface. But the liquid surface will have the tendency to have minimum potential energy. So a minimum number of molecules remains on the surface of the liquid.
Thus the free surface of a liquid behaves like a stretched elastic membrane and has a tendency to contract so as to minimize its surface area.
Definition of Surface Tension:
Surface tension or force of surface tension is the force per unit length of an imaginary line drawn in any direction on the free surface of a liquid, the line of action of the force being on the surface and at right angles to the length of the imaginary line.
Dimensions and Units of Surface tension:
Surface Tension (T) = Force (F) / Effective Length (L)
∴ [T] = [F] / [L]
∴ [T] = [L1M1T-2] / [L1]
∴ [T] = [L0M1T-2]
∴ S.I. unit of T = S.I. unit of F /S.I. unit of L
∴ S.I. unit of T= newton (N) / metre (m)
Thus the dimensions of surface tension are [L0M1T-2] and its S.I. unit is N/m and c.g.s. unit is dyne/cm.
Factors Affecting Surface Tension:
Temperature:
The surface tension at t °C is given by
T = T0 (1 – α t)
Where T0 = Surface tension at 0° C and α = Temperature coefficient of surface tension. Its value depends on the nature of the liquid.
- Generally, the surface tension of liquid decreases with an increase in temperature. it is due to the increase in the kinetic energy of liquid molecules.
- The temperature at which surface tension of a liquid becomes zero is called a critical temperature of the liquid.
- The surface tension of a liquid at the boiling point and at critical temperature is zero.
- The surface tension of molten cadmium and copper increases with an increase in temperature.
Impurities:
If the impurity added in a liquid is highly soluble in it, then the surface tension of liquid increases. If the impurity added in a liquid is partly soluble in it, then it decreases.
Contamination:
The presence of dust particles in liquid decreases its surface tension.
Next Topic: Surface Tension in Everyday Life
One reply on “Surface Tension”
very good work