Science > Chemistry > Chemical Equilibrium > Physical Equilibrium
There are two types of equilibrium: a) physical equilibrium and b) chemical equilibrium. In this article, we shall study physical equilibrium.
State of Equilibrium:
In many chemical reactions, it is observed that they do not proceed to completion when they are carried out in a closed container. That means in such reactions the reactants are not completely converted into products.
Initially, the concentration of the reactants decreases. After some time the concentration of the reactants stops decreasing and it appears that reaction is stopped. This state of the system in which no further net change occurs is called the state of equilibrium. There are two types of equilibria. a) Physical equilibrium and b) Chemical equilibrium.
The equilibrium attained in physical processes is called physical equilibrium. e.g. Equilibrium achieved in physical processes like the dissolution of salt or evaporation of water etc.
The equilibrium attained in chemical processes is called chemical equilibrium. e.g. Equilibrium achieved in chemical processes like decomposition of calcium carbonate, the reaction between hydrogen and iodine etc.
Types of Physical Equilibrium:
The change of a substance from one phase to another phase is called a physical process. The equilibrium attained in physical processes is called physical equilibrium.
Solid – Liquid Equilibrium
Example: Ice(s) ⇌ Water(l) . It is a physical equilibrium because no chemical reaction is involved.
For any pure substance at atmospheric pressure, the temperature at which the solid and liquid can coexist is called as a normal melting point of the solid and for any pure substance at atmospheric pressure, the temperature at which the solid and liquid can coexist is called as a normal freezing point of the liquid.
When a pure solid is heated it starts transforming into a liquid at a certain temperature (melting point of the solid). The process is called the melting of solid. At this temperature, both the solid and the liquid state of the substance coexist under the given conditions of pressure.
At this temperature, the solid-state of a substance is in equilibrium with the liquid state of a substance. If this mixture is taken in a well-insulated container then this constitutes a system in which solid is in dynamic equilibrium with the liquid.
At such point, the interconversion between solid-state and the liquid state takes place continuously. It is not stopped. actually, the number of molecules of solid getting converted into the liquid is equal to the number of molecules of liquid getting converted into solid. Thus the mass of the solid and mass of the liquid in the system remains constant. This represents the dynamic equilibrium between the solid and liquid.
At this stage, The rate of melting = The rate of freezing
Liquid – Vapour Equilibrium:
Example: water(l) ⇌ Steam(g) It is a physical equilibrium because no chemical reaction is involved.
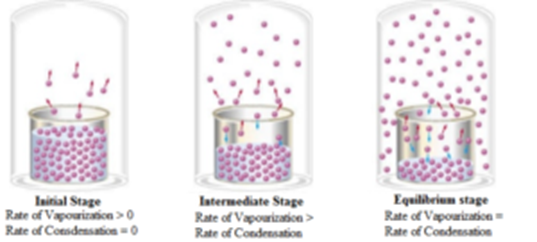
Let us consider evaporation of water in a closed vessel fitted with a mercury pressure gauge. At room temperature the evaporation of water starts, gradually the quantity of vapours in the vessel increases and pressure called a vapour pressure builds up. Due to this gradual increase in the pressure is indicated by the manometer.
A stage is reached when the manometer shows a constant reading of the vapour pressure. Showing no more evaporation of the water in the vessel. But it is not the case. Actually, the rate of evaporation of water is equal to the rate of condensation of the vapours. It shows there is an equilibrium between the two states.
At this stage, the rate of evaporation = The rate of condensation
The equilibrium between the vapours and the liquid is attained only in a closed vessel. If the vessel is open, the vapours leave the vessel and get dispersed. As a result, the rate of condensation can never become equal to the rate of evaporation.
The vapour formed has more volume to occupy due to an increase in the volume. Hence initially vapour pressure decreases due to an increase in the volume. Due to more availability of the volume, the rate of evaporation increases and that of condensation decreases. The vapour pressure does not depend upon the size of the vessel containing it. It is constant at a given temperature. Thus at equilibrium, the starting vapour pressure is restored. Similarly, at the equilibrium, the rate of evaporation of the liquid is equal to the rate of condensation of its vapours.
Solid – Gas (Vapours) Equilibrium:
Example: NH4Cl(s) NH4Cl(g)
This type of equilibrium is observed in sublimable substances.
One reply on “Physical Equilibrium”
thank you, this article helped my understanding immensely!