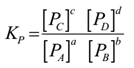Science > Chemistry > Chemical Equilibrium > Applications of Le-Chatelier’s Principle In this article we shall study the application of Le-Chatelier’s principle in Haber’s process, contact proces, etc. Statement of Le-Chatelier’s Principle: This principle is given by, a French chemist Le-Chatelier in 1888. It states that “If an external stress is applied to a reacting […]
Categories
Applications of Le-Chatelier’s Principle
- Post author By Hemant More
- Post date April 2, 2020
- 4 Comments on Applications of Le-Chatelier’s Principle
- Tags Chemical equilibrium, Chemical reaction, Chemistry, Contact process, Endothermic reaction, Equilibrium, Equilibrium constant., Exothermic reaction, Haber's process, Heterogeneous reaction, Homogeneous reaction, Irreversible reaction, Le-Chatelier's principle, Manufacturing of ammonia, Manufacturing of nitric oxide, Manufacturing of ozone, Manufacturing of sulphur trioxide, Physical equilibrium, Products, Reactants, Reversible reaction