Science > Chemistry > Chemical Equilibrium > More Examples of Physical Equilibrium
In this article, we shall study more examples of physical equilibrim and the dynamic nature of equilibrium.
Dissolution of solids in liquids:
It is not possible to dissolve just any amount of a solute in a given amount of solvent. A stage will be reached when no more salt can be dissolved. A solution in which no more solute can be dissolved is called a saturated solution.
The amount of solute required to prepare a saturated solution in a given quantity of a solvent at given temperature is known as the solubility of the solute at that temperature. The saturated solution corresponds to the state of physical equilibrium.
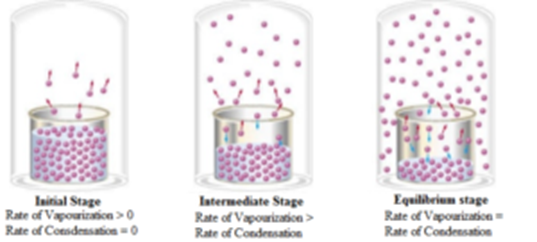
When a solid (say sugar) is dissolved in a liquid (say water) then due to molecular vibrations the molecules on the surface of the crystal leaves, the crystal and start moving in the solvent freely. At the same time the molecule which already left the crystal return back to the crystal. Initially, the rate of leaving of the molecule from the crystal is much greater than their rate of returning to the crystal.
As the number of molecules in the solution increases the rate leaving of molecules from crystal decreases while the rate of returning of molecules to the crystal increases. A stage is reached when the rate of leaving of the molecules from the crystal surface (the rate of dissolution) is equal to the rate of returning of the molecules to the crystal surface (the rate of precipitation). Thus equilibrium state is attained.
Thus at equilibrium, Sugar(in solution) ⇌ Sugar(solid)
Dynamic Nature of Equilibrium of Dissolution of Solid in Liquid:
The dynamic nature of equilibrium of dissolution of a solid in the liquid can be experimentally demonstrated by dissolving radioactive sugar (containing radioactive carbon) into a saturated solution of non-radioactive sugar. After some time it is observed that the solution becomes radioactive, while the quantity of non-dissolved sugar remains the same.
It clearly indicates that in saturated solution radioactive sugar is getting dissolved into the solution at the same time non-radioactive sugar is getting precipitated out. Thus even if the process of dissolution seems to be stopped, actually both dissolution and precipitations are going on such that their rates are equal. This experiment demonstrates the dynamic nature of equilibrium of the dissolution of the solid in a liquid.
Effect of Increase in Temperature on Solubility of Solid in Liquid:
Solid molecules are at fixed positions in their crystal lattice. When solid is dissolved in liquid, the molecules of the solid acquire randomness. Thus the kinetic energy of the molecules of the solid after dissolving in liquid increases. This increase in kinetic energy is due to absorption of heat from the system. Thus dissolution of a solid in a liquid is an endothermic process.
By Le-Chatelier’s principle, if we increase the temperature, then the reaction proceeds in a direction to decrease the temperature. Hence the forward reaction is favoured. Hence the increase in the temperature of the endothermic reaction increases the rate of reaction. Hence the solubility of a solid in liquid increases with increase in temperature
Dissolution of Gases in Liquids:
The best example of this type of equilibrium is in soda water. When the bottle is opened the carbon dioxide gas dissolved in it fizzles out rapidly.
By Henry’s law “The mass of a gas dissolved in a given mass of a solvent, at a given temperature, is directly proportional to the pressure of the gas above the solvent”. In sealed soda water bottle the carbon dioxide gas is filled with high pressure. Due to the high-pressure appreciable amount of the gas is dissolved in water. When the cap is opened the gas pressure above the solution decreases and the gas dissolved under pressure fizzes out of the solution to attain new equilibrium state. In the case of gas in liquid solution, the solubility of the gas in liquid decreases with increase in the temperature.
The pressure exerted by the vapors in equilibrium with liquid at a particular temperature is called a vapor pressure of the liquid at that temperature.
Henry’s Law:
This law explains the effect of pressure on the solubility of a gas in a liquid. It states that “The mass of a gas dissolved in a given mass of a solvent, at a given temperature, is directly proportional to the pressure of the gas above the solvent”.
Explanation: If ‘m’ is the mass of the gas dissolved in the solvent and ‘p’ is the pressure of the gas above the solvent then
m α p i.e. m = kP
Effect of Increase in Temperature on Solubility of Gas (Carbon dioxide) in Liquid (Water):
Gas molecules are always in the state of random motion. When gas is dissolved in water, the randomness of molecules decreases. Thus the kinetic energy of the molecules of the gas after dissolving in liquid decreases. This decrease in kinetic energy results in the evolution of heat. Thus dissolution of a gas in a liquid is an exothermic process.
By Le-Chatelier’s principle, if we increase the temperature, then the reaction proceeds in a direction to decrease the temperature. Hence the backward reaction is favoured. Hence the increase in the temperature of exothermic reaction decreases the rate of reaction. Hence the solubility of a gas in water decreases with increase in temperature
Characteristics of Physical Equilibrium:
- In the case of Liquid Gas equilibrium, the vapour pressure of the liquid is constant at equilibrium at a particular temperature.
- For Solid Liquid equilibrium, there is only one temperature at which the two phases can co-exist at a particular pressure. This temperature is known as the melting point of the solid or freezing point of the liquid.
- For dissolution of solids in liquids, the solubility is constant at a given temperature.
- For dissolution of gases in liquids, the concentration of a gas in a liquid, at a given temperature is directly proportional to the pressure of the gas over the liquid.
- At physical equilibrium, the measurable properties of the system become constant.
- At physical equilibrium, there is a dynamic balance between the two opposite processes.
- The equilibrium is attained only in a system which cannot gain matter from the surroundings or lose matter to the surroundings. i.e. the system should be a closed system.
- When the equilibrium is attained, there exists an expression involving the concentration of substances involved in equilibrium which reaches a constant value at a given temperature. For the dissolution of carbon dioxide in water, the following expression has a constant value.
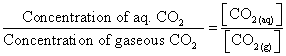
- The magnitude of the constant value of the concentration-related expression indicates the extent to which the process proceeds before reaching equilibrium. In above expression if the value of the ratio is higher then the numerator should be greater, hence more carbon dioxide gas is dissolved in water.