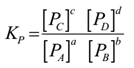Science > Chemistry > Chemical Equilibrium > Writing Expression for Equilibrium Constant
In this article, we shall stuudy to write expression for equilibrium constant.
Steps Involved in Writing Expression for Equilibrium Constant of a Reaction:
- Write the balanced chemical equation for the reaction.
- Write the products of equilibrium concentrations of the products in the numerator. Omit pure solids, pure liquids and the solvents in dilute solutions.
- Write the products of equilibrium concentrations of the reactants in the denominator. Omit pure solids, pure liquids and the solvents in dilute solutions.
- Raise each concentration term to the power equal to stoichiometric coefficients of the species in the equation.
Homogeneous Equilibrium:
The equilibrium in which all the substances involved exist in a single homogeneous phase is called homogeneous equilibrium.
Examples:
N2(g) + 3 H2(g) ⇌ 2NH3(g)
H2(g) + I2(g) ⇌ 2HI(g)
2SO2(g) + O2(g) ⇌ 2SO3(g)
NH3(aq) + H2O(l) ⇌ 2NH4+(aq) + OH-(aq)
2N2O(g) ⇌ 2N2(g) + O2(g)
CH3COOC2H5(aq) + H2O(l) ⇌ CH3COOH(aq) + C2H5OH(aq)
Heterogeneous Equilibrium:
The equilibrium in which the substance involved are present in different phases is called heterogeneous equilibrium.
Examples:
CaCO3(s) ⇌ CaO(s) + . CO2(g)
CaCO3(s)+H2O(l)+CO2(g) ⇌ Ca2+(aq)+ 2HCO3–(aq)
(NH4)2CO3(s) ⇌ 2NH3(g) + CO2(g)+ H2O(g)
2Mg(s) + O2(g) ⇌ 2MgO(s)
Note: Pure liquids and solids are ignored while writing the equilibrium constant expression.
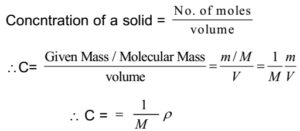
Now, for pure solids and pure liquids, the molar mass M and density ρ are constant. Hence the concentration remains constant.
- When pure solids are involved in equilibrium, its concentration remains constant no matter how much of it is present. Therefore by convention, the concentrations of all solids involved in equilibrium are taken as unity.
- When pure liquids are involved in equilibrium, its concentration remains constant no matter how much of it is present. Therefore by convention, the concentrations of all liquids involved in equilibrium are taken as unity.
- For equilibria in the aqueous medium, the concentration of solvent (water) will not change appreciably because it is present in large excess. Hence by convention, the concentration of solvent (water) is taken as unity.
- Hence in general, pure liquids, pure solids, and solvents can be ignored while writing the equilibrium constant expression.
Writing the Expression for Kc and Kp:
BaCO3(s) ⇌ BaO(s) + CO2(g)

4NH3(g) + 5O2(g) ⇌ 4NO(g)+ 6H2O(g)

NH3(g) + HCl(g) ⇌ NH4Cl(s)

3Cl2(g) + 2NO2(g) ⇌ 2NO2Cl3(g)

Fe3+(aq) + SCN–(aq) ⇌ FeNCS2+(aq)

CH4(g) + 2H2S(g) ⇌ CS2(g)+ 4H2(g)

MgCO3(s) ⇌ MgO(s) + CO2(g)

AgBr(s) ⇌ Ag+(aq)+ Br–(aq)
Kc = [Ag+][Br –]
CH3COCH3(l) ⇌CH3COCH3(g)
Kc = [CH3COCH3(g)]
CH4(g) + 2O2(g) ⇌ CO2(g)+ 2H2O(g)

Al(s) + 3H+(aq) ⇌ Al3+(aq)+ 3/2H2(g)

HPO42-(aq) + H2O(l) ⇌ H3O+(aq)+ PO43-(aq)

Ag2O(s) + 2HNO3(aq) ⇌ 2AgNO3(aq)+ H2O(l)

Ni(s) + 4CO(g) ⇌ Ni(CO)4(g)

CuO(s) + H2(g) ⇌ Cu(s)+ H2O(g)

N2(g) + 3H2(g) ⇌ 2NH3(g)

Fe3+(aq) + 3OH–(aq) ⇌ Fe(OH)3(s)

2N2O(g) ⇌ 2N2(g) + O2(g)

C(s)+ CO2(g) ⇌ 2CO(g)

Consider a hypothetical reversible reaction involving homogeneous gaseous phase
aA(g) + bB(g) ⇌ cC(g) + dD(g)
The equilibrium constant in terms of the partial pressure is given by


This is the relation between Kc and Kp.
Where Δn = (Sum of the exponents in the numerator of concentration quotient) – (Sum of the exponents in the denominator of concentration quotient)
Steps Involved in Finding Relation Between Kc and Kp :
- Write the balanced chemical equation for the reaction.
- Find the change in the number of moles of gaseous species by the following formula.

- Now, use the relation

Examples to Find Relation between Between Kc and Kp :
Example – 01:

Example – 02:

Example – 03:

Example – 04:
For the reaction N2(g) + 3H2(g) ⇌ 2NH3(g), the value of the equilibrium constant Kp is 3.6 x 10-2 at 500 K. Calculate the value of Kc for the reaction at the same temperature. Take R = 0.0831 bar lit mol-1 K-1.

Example – 05:
For the reaction 2SO2(g)) + O2(g) ⇌ 2SO3(g), the value of the equilibrium constant Kp is 2.0 X 1010 bar-1 at 450 K. Calculate the value of Kc for the reaction at the same temperature. Take R = 0.0831 bar lit mol-1 K-1.

Example – 06:
For the reaction 2NOCl(g) ⇌ 2NO(g)+ Cl2(g), the value of the equilibrium constant Kp is1.8 X 10-2 at 500 K. Calculate the value of Kc for the reaction at the same temperature. Take R = 0.0831 bar lit mol-1 K-1.

Example – 07:
For the reaction CaCO3(s) ⇌ CaO(s)+ CO2(g), the value of equilibrium constant Kp is 167 at 1073 K. Calculate the value of Kc for the reaction at the same temperature. Take R = 0.0831 bar lit mol-1 K-1.

Example – 08:
Find the ratio of Kp/Kc for the reaction, CO(g) + 1/2O2(g) ⇌ 2CO2(g), at 500 K. Take R = 0.0831 bar lit mol-1 K-1.

Example – 09:
Find the ratio of Kp/Kc for the reaction, N2(g) + O2(g) ⇌ 2NO(g)

Example – 10:
The equilibrium constant Kp for the reaction H2(g) + I2(g) ⇌ 2HI(g) is 130 at 510 K. Calculate Kc for following reactions a) 2HI(g) ⇌ H2(g) + I2(g), b) HI(g) ⇌ 1/2H2(g) + 1/2I2(g), c) 1/2H2(g) + 1/2I2(g) ⇌ HI(g).

∴ Kc = 130

For the reaction 2HI(g) ⇌ H2(g) + I2(g),

For the reaction, HI(g) ⇌ 1/2H2(g) + 1/2I2(g)

For reaction, 1/2H2(g) + 1/2I2(g) ⇌ HI(g).