Science > Chemistry > Chemical Equilibrium > Chemical Equilibrium
Rate of a Chemical Reaction:
Rate of reaction is the quantities of the reactants converted into the products in unit time. Or Number of moles of the reactants used up or the number of moles of the products formed per unit time.
Mathematically it can be expressed as,
Rate of Reaction = No. of moles of reactants consumed / Time
Thus, the rate of chemical reaction is the change in concentration of the reactants in unit time.
Active Mass:
Active mass is molar concentration per unit volume of that substance. It is denoted by enclosing the symbol or formula of that substance in square bracket and expressed in ” moles/ litres”.
Active Mass = No. of moles of substance/Volume in litres
Demonstration of Chemical Equilibrium:
Hydrogen and iodine are heated in a closed vessel. The reacting mixture is deep violet in colour due to the presence of iodine vapours. At 717 K the reaction between the reactants takes place. Gradually the deep violet colour of the mixture becomes faint indicating that iodine is being consumed. After some time it is observed that the intensity of violet colour becomes constant, indicating that the reaction is stopped although both hydrogen and iodine are present. This happens because equilibrium is reached. Thus the reaction is reversible and can be represented by
H2(g) + I2(g) ⇌ 2HI(g)
Chemical Equilibrium:
Chemical equilibrium is a state of a system of reacting substances at which the rate of the forward reaction is equal to the rate of backward reaction.
Explanation:
Consider general reversible reaction
A + B ⇌ C + D
At the start of the reaction, only reactants are present. Hence concentrations of A and B are maximum and that of C and D are minimum (zero). Hence the rate of the forward reaction is maximum and that of the backward reaction is zero. As the reaction proceeds reactants A and B react to produce products C and D. Thus the concentrations of reactants A and B decrease and that of products increases. Thus the rate of the forward reaction decreases and that of the backward reaction increases.
A point will be reached when the rate of the forward reaction is equal to the rate of the backward reaction. This state of the reaction is called the chemical equilibrium. At chemical equilibrium concentration of reactants and product remains unchanged throughout. It means that at equilibrium neither forward nor backward reaction stops, but are continued at equal rates in opposite directions. Hence we can say that the equilibrium is dynamic and not static. It is to be noted that the reversible reactions involving gaseous substances are carried out in a closed vessel.
Graphical Representation:
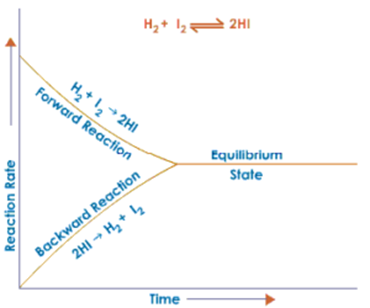
Chemical Equilibrium a Dynamic Equilibrium:
- Chemical equilibrium is a state of a system of reacting substances at which the rate of the forward reaction is equal to the rate of the backward reaction
- At chemical equilibrium concentration of reactants and products remains unchanged throughout. It means that at equilibrium neither forward nor backward reaction stops, but are continued at equal rates in opposite directions.
- The concentrations of reactants and products at chemical equilibrium are constant. At the same time, all the observable properties of the system become constant. Hence we can say that the equilibrium is dynamic and not static.
Characteristics of Chemical Equilibrium:
- Chemical equilibrium exists in reversible reactions only.
- At equilibrium, the rate of the forward reaction is equal to the rate of the backward reaction.
- At equilibrium, the concentrations of reactants and products remain constant. These concentrations are called equilibrium concentrations.
- At equilibrium, both the forward reaction and the backward reaction do not stop. Actually, all the reactants and products are present at the equilibrium
- At equilibrium, neither the forward reaction nor the backward reaction has ceased. Both reactions proceed in opposite directions with an equal rate. Thus chemical equilibrium is dynamic in nature.
- At the chemical equilibrium, the observable properties of the system like pressure, colour, concentrations, etc. become constant and remain unchanged thereafter.
- The equilibrium can be approached from either direction.
- Equilibrium can only be attained only if the system is closed.
- State of chemical equilibrium is unaffected by catalyst because the presence of catalyst equally increases the speed of both the forward and the backward reaction.
- State of chemical equilibrium changes with the change in factors like concentration, temperature and pressure.
Factors Affecting Chemical Equilibrium:
Effect of the Change of Concentration:
If the concentration of reactants increases then there will be a rise in the concentration of the products and thus equilibrium is shifted from left to right.
Explanation: By the law of mass action, the rate of a chemical reaction is directly proportional to the product of active masses of reactants, at given temperature at that instant. As the concentration of reactants is increased, the product of concentrations of reactants increases thus to keep the value of equilibrium constant the same the product of concentrations of products is increased. Thus more products are formed. Hence equilibrium is shifted from left to right.
Effect of the Change of Pressure:
Change in pressure plays an important role in gaseous reactions. There can be three types of gaseous reactions:
- Chemical reactions accompanied by the increase in volume
- Chemical reactions accompanied by the decrease in volume
- Chemical reactions accompanied by no change in volume
Chemical reactions accompanied by the increase in volume:
Consider reaction.
PCI5(g) ⇌ PCl3(g) + Cl2(g)
1 Vol 1 Vol 1 Vol
1 Vol 2 Vol
In this reaction, 1 volume of reactants gives 2 volumes of products. Thus in this reaction volume is increased.
The chemical reactions involving gases and accompanied by the increase in volume are favoured by a reduction in pressure. Thus by decreasing the pressure at equilibrium, equilibrium is shifted towards the right.
Chemical reactions accompanied by the decrease in volume:
Consider the reaction.
N2(g) + 3 H2(g) ⇌ 2NH3(g)
1 Vol 3 Vol 2 Vol
4 Vol 2 Vol
In this reaction, 4 volumes of reactants give 2 volumes of products. Thus in this reaction volume is decreased.
Chemical reactions involving gases and accompanied by a decrease in the volume are favoured by an increase in pressure. Thus by increasing the pressure at equilibrium, equilibrium is shifted towards the right.
Chemical reactions accompanied by no change in volume:
Consider following reaction.
H2(g) + Cl2(g) ⇌ 2HCl(g)
1 Vol 1 Vol 2 Vol
2 Vol 2 Vol
In this reaction, 2 volumes of reactants give 2 volumes of products. Thus in this reaction volume is not changed.
Chemical reactions involving gases and accompanied by no change in volume are not affected by the change in pressure.
Effect of the Change of Temperature:
If the temperature of the exothermic chemical reaction is increased, then the concentration of products reduces and thus the equilibrium is shifted towards left. Hence the reduction in temperature favours exothermic reaction at equilibrium and increase in temperature favours endothermic reaction.
It is to be noted that in a reversible reaction if one reaction is exothermic then another reaction is endothermic. Thus the effect of change of temperature on the two reactions is different.
Effect of Presence of a Catalyst:
A catalyst is a substance which increases or decreases the rate of a reaction without taking part in the chemical reaction.
In a reversible reaction at equilibrium, catalyst affects the rate of both the forward reaction and the backward reaction to the same extent. Hence catalyst at equilibrium does not affect chemical equilibrium.
Addition of Inert Gas at Constant Volume:
If the inert gas is added to the reaction at constant volume It will result in the increase of the total pressure of the system. At the start, the partial pressures of the reactants and pressure will get changed. But in short time they will attain their equilibrium values before addition of the inert gas. Thus partial pressures of reactants and products are not getting affected. Hence there is no effect of the addition of inert gas on equilibrium at constant volume.
Addition of Inert Gas at Constant Pressure:
If the inert gas is added to the reaction at constant pressure It will result in an increase in the volume of the system. Thus the concentration of the reactants and products will decrease. To counterbalance this stress (change) the equilibrium will shift to the side where the number of moles is increased.
2 replies on “Chemical Equilibrium”
Very well summarize and quite comprehensible.
Gracias
Very well and good explanation on factors affecting equilibrium