Science > Chemistry > Chemical Equilibrium > Le-Chatelier’s Principle
In this article, we shall study Le-Chatelier’s principle with examples.
Statement:
This principle is given by, a French chemist Le-Chatelier in 1888. It states that “If an external stress is applied to a reacting system at equilibrium, the system will adjust itself in such a way that the effect of the stress is nullified”.
Explanation:
Let us consider a general reversible reaction.
A + B ⇌ C + D
The equilibrium constant for the reaction is given by
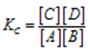
If the concentration of any one reactant say A is increased then by Le-Chatelier’s Principle the forward reaction should be favoured so that the increase in the concentration of A is nullified. It can be explained as follows. As the concentration of reactant, A increases the denominator of mass equation increases. To keep the value of the equilibrium constant the same the value of Kc constant the numerator should increase. This is possible only if the concentration of C and D is increased. It is possible if more and more C and D are formed thus the reaction proceeds in the forward direction. i.e. forward reaction is favoured.
If the concentration of any one product, say C is increased then by Le-Chatelier’s Principle the backward reaction should be favoured so that the increase in the concentration of C is nullified. It can be explained as follows. As the concentration of product C increases the numerator of mass equation increases. To keep the value of Kc, the denominator should increase. This is possible only if the concentration of A and B is increased. It is possible if more and more A and B are formed thus the reaction proceeds in the backward direction. i.e. backward reaction is favoured.
Effect of the Change of Concentration on the chemical Equilibrium:
According to Le-Chatelier’s principle, when the concentration of one of the substance in a system in equilibrium is increased, then the equilibrium will shift so as to use up the substance added.
Explanation Using Le-Chatelier’s Principle:
Let us consider a general reversible reaction.
A + B ⇌ C + D
The equilibrium constant for the reaction is given by
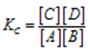
If the concentration of any one reactant say A is increased then by Le-Chatelier’s Principle the forward reaction should be favoured so that the increase in the concentration of A is nullified. It can be explained as follows. As the concentration of reactant, A increases the denominator of mass equation increases. To keep the value of the equilibrium constant the same the value of Kc constant the numerator should increase. This is possible only if the concentration of C and D is increased. It is possible if more and more C and D are formed thus the reaction proceeds in the forward direction. i.e. forward reaction is favoured.
If the concentration of any one product say C is increased then by Le-Chatelier’s Principle the backward reaction should be favoured so that the increase in the concentration of C is nullified. It can be explained as follows. As the concentration of product C increases the numerator of mass equation increases. To keep the value of Kc, the denominator should increase. This is possible only if the concentration of A and B is increased. It is possible if more and more A and B are formed thus the reaction proceeds in the backward direction. i.e. backward reaction is favoured.
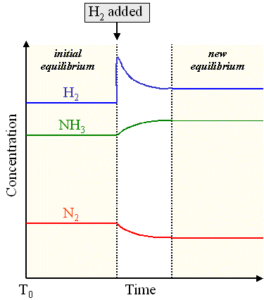
The following graph shows the variation in concentration of the species on increasing the concentration of hydrogen in a reaction to produce ammonia from nitrogen and hydrogen.
Everyday Life Examples to Explain the Effect of the Change of Concentration on the Equilibrium:
Clothes dry quicker when there is a breeze.
Due to breeze or by shaking clothes in the air, the water vapours in the nearby air are removed or carried away. To establish the equilibrium the water from wet clothes starts evaporating. Thus they get dried fast.
On a humid day, we sweat more
Our body is losing water continuously by forming sweat. On a normal day, this sweat gets evaporated as soon as it is formed on the surface of the body. On a humid day the surrounding air contains a large amount of water vapours. Hence our body cannot lose water in the form of vapours. Thus the water remains on our body as sweat.
Transfer of oxygen by haemoglobin in the blood.
Haemoglobin is a protein present in red blood corpuscles which act as an oxygen carrier. The equilibrium is represented as
Hb(s) + O2(g) ⇌ HbO2(s)
In lungs, there is an equilibrium of this reaction, when HbO2 (oxyhaemoglobin) reaches the site of tissues where the partial pressure is low. Now the equilibrium adjusts itself by shifting towards the right by releasing oxygen from oxyhaemoglobin. When the blood returns back to the lungs, where the partial pressure is higher, more oxyhaemoglobin is formed.
Removal of CO2 from tissues
At the site of the tissues, the partial pressure of carbon dioxide is high. It dissolves into the blood and carried to lungs. In lungs partial pressure of carbon dioxide is low and it gets released from the blood.
Effect of the Pressure on the Chemical Equilibrium:
Change in pressure plays an important role in gaseous reactions. The change of pressure has effect only on those equilibria which involves gaseous substances. There can be three types of gaseous reactions:
- Chemical reactions accompanied by an increase in volume
- Chemical reactions accompanied by a decrease in volume.
- Chemical reactions accompanied by no change in volume.
By Le-Chatelier’s principle, at a constant temperature, increase in pressure will favour a reaction which is accompanied by a decrease in volume and decrease in pressure will favour a reaction which is accompanied by the increase in volume.
Chemical reactions accompanied by an increase in volume:
Consider following reaction.
PCl5(g) ⇌ PCl3(g) + Cl2(g)
1 Vol 1 Vol 1 Vol
1 Vol 2 Vol
In this reaction, 1 volume of reactants gives 2 volumes of products. Thus in this reaction volume is increased.
Chemical reactions involving gases and accompanied by an increase in volume are favoured by a reduction in pressure. Thus by decreasing the pressure at equilibrium, equilibrium is shifted towards the right.
Chemical reactions accompanied by a decrease in volume:
Consider following reaction.
N2(g) + 3H2(g) ⇌ 2 NH3(g)
1 Vol 3 Vol 2 Vol
4 Vol 2 Vol
In this reaction, 4 volumes of reactants give 2 volumes of products. Thus in this reaction volume is decreased.
Chemical reactions involving gases and accompanied by a decrease in the volume are favoured by an increase in pressure. Thus by increasing the pressure at equilibrium, equilibrium is shifted towards the right.
Chemical reactions accompanied by no change in volume:
Consider following reaction.
H2(g) + I2(g) ⇌ 2 HI(g)
1 Vol 1 Vol 2 Vol
2 Vol 2 Vol
In this reaction, 2 volumes of reactants give 2 volumes of products. Thus in this reaction volume is not changed.
Chemical reactions involving gases and accompanied by no change in volume are not affected by the change in pressure.
Effect of Temperature on the Chemical Equilibrium:
If the temperature of the exothermic chemical reaction is increased, then the concentration of products reduces and thus the equilibrium is shifted towards left. Hence the reduction in temperature favours exothermic reaction at equilibrium. and increase in temperature favours endothermic reaction.
It is to be noted that in a reversible reaction if one reaction is exothermic then another reaction is endothermic. Thus the effect of change of temperature on the two reactions is different.
By Le-Chatelier’s principle. for an exothermic reaction at equilibrium lowering of temperature will favour the forward reaction And for an endothermic reaction, an increase in temperature will favour the forward reaction.
Effect of the Catalyst on the Chemical Equilibrium:
The catalyst is a substance which increases or decreases the rate of a reaction without taking part in the chemical reaction. In a reversible reaction at equilibrium, catalyst affects the rate of both forward reaction and backward reaction by the same extent. Hence catalyst at equilibrium does not affect chemical equilibrium.
Effect of Inert Gas Addition:
If the volume is kept constant and an inert gas such as argon is added which does not take part in the reaction, the equilibrium remains undisturbed. It is due to the fact that the addition of an inert gas at constant volume does not change the partial pressure or the molar concentration of substances involved in the reaction. The change will take place if and only if the added gas is a reactant or product involved in the reaction.