Science > Chemistry > Everyday Chemistry > Cleansing Agents: Soaps and Detergents
In the last article, we have studied the use of chemicals in food is in the form of food additives, food preservatives, artificial sweeteners, and antioxidants. In this article, we shall study about cleansing agents: Soap and detergent
Soaps:
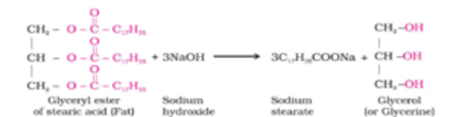
Cleansing agents soaps and synthetic detergents improve the cleansing properties of water. These help in the removal of fats that bind other materials to the fabric or skin. They are used for cleaning purposes and are sodium or potassium salts of long-chain fatty acids, e.g., stearic, oleic, and palmitic acids.
Only sodium and potassium soaps are soluble in water and are used for cleaning purposes. Generally, potassium soaps are soft to the skin than sodium soaps. These can be prepared by using potassium hydroxide solution in place of sodium hydroxide.
Soaps containing sodium salts are formed by heating fat (i.e., a glyceryl ester of fatty acid) with aqueous sodium hydroxide solution. This reaction is known as saponification. In this reaction, esters of fatty acids are hydrolyzed and the soap obtained remains in colloidal form. It is precipitated from the solution by adding sodium chloride. The solution left after removing the soap contains glycerol, which can be recovered by fractional distillation.
Types of Soaps and Their Preparations:
Basically, they are made by boiling fats or oils with suitable soluble hydroxide. Variations are made by using different raw materials.
- Toilet soap is prepared by using better grades of fats and oils and care is taken to remove excess alkali. Colour and perfumes are added to make these more attractive.
- Soaps that float in water are made by beating tiny air bubbles before their hardening.
- Transparent soaps are made by dissolving the soap in ethanol and then evaporating the excess solvent.
- In medicated soaps, substances of medicinal value are added. In some soaps, deodorants are added.
- Shaving soaps contain glycerol to prevent rapid drying. A gum called, rosin is added while making them. It forms sodium rosinate which lathers well.
- Laundry soaps contain fillers like sodium rosinate, sodium silicate, borax and sodium carbonate.
- Soap chips are made by running a thin sheet of melted soap onto a cool cylinder and scraping off the soaps in small broken pieces. Soap granules are dried miniature soap bubbles. Soap powders and scouring soaps contain some soap, a scouring agent (abrasive) such as powdered pumice or finely divided sand, and builders like sodium carbonate and trisodium phosphate. Builders make the soaps act more rapidly.
Why do soaps not work in hard water?
Hard water contains calcium and magnesium ions. These ions form insoluble calcium and magnesium soaps respectively when sodium or potassium soaps are dissolved in hard water. These insoluble soaps separate as scum in water and are useless as a cleansing agent. In fact, these are hinderance to good washing because the precipitate adheres to the fibre of the cloth as gummy mass.
Hair washed with hard water looks dull because of this sticky precipitate. Dye does not absorb evenly on cloth washed with soap using hard water, because of this gummy mass.
Detergents:
Detergents are sodium salts of long chain alkyl sulphates or a long chain of alkyl benzene sulphonate. They can be used both in soft and hard water as they give foam even in hard water. Some of the detergents give foam even in ice cold water.
Classification of Synthetic Detergents:
Synthetic detergents are mainly classified into three categories: Anionic detergents, Cationic detergents and Non-ionic detergents
Anionic Detergents:
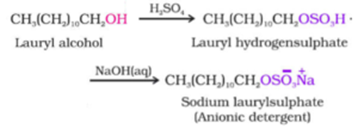
Anionic detergents are sodium salts of sulphonated long-chain alcohols or hydrocarbons. Example: Sodium n-dodecyl benzene sulphonate
Alkyl hydrogen sulphates formed by treating long chain alcohols with concentrated sulphuric acid and neutralized with alkali to form anionic detergents.
Similarly, alkyl benzene sulphonates are obtained by neutralising alkyl benzene sulphonic acids with alkali.
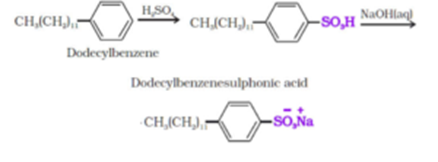
In anionic detergents, the anionic part of the molecule is involved in the cleansing action. Sodium salts of alkyl benzene sulphonates are an important class of anionic detergents. They are mostly used for household work. Anionic detergents are also used in toothpaste.
Cationic Detergents:
Cationic detergents are quarternary ammonium salts of amines with acetates, chlorides or bromides as anions. The cationic part possesses a long hydrocarbon chain and a positive charge on the nitrogen atom. Hence, these are called cationic detergents. Example: Cetyltrimethylammonium bromide. It is a popular cationic detergent and is used in hair conditioners.

Cationic detergents have germicidal properties and are expensive, therefore, these are of limited use.
Non-ionic Detergents:
Non-ionic detergents do not contain any ion in their constitution. Example: Pentaerythrityl stearate
One such detergent is formed when a stearic acid reacts with polyethene glycol.

Liquid dishwashing detergents are a non-ionic type.
Mechanism of cleansing action of this type of detergents is the same as that of soaps. These also remove grease and oil by micelle formation.
Ecological Problem with Detergents:
The main problem that appears in the use of detergents is that if their hydrocarbon chain is highly branched, then bacteria cannot degrade them easily. Their slow degradation leads to their accumulation. Effluents containing such detergents reach the rivers, ponds, etc. These persist in water even after sewage treatment and cause foaming in rivers, ponds and streams and their water get polluted. These days the branching of the hydrocarbon chain is controlled and kept to the minimum. Unbranched chains can be biodegraded more easily and hence pollution is prevented.
Cleansing Action of Soaps and Detergents:
Soaps and detergents have two parts, the hydrophobic part and the hydrophilic part.

Hydrophobic or water repelling non-polar part (usually a long hydrocarbon chain) is soluble in oil and greases but insoluble in water. Hydrophilic or water attracting polar parts such as carboxylic group or sulphonate or sulphate is soluble in water and insoluble in oil and greases.
Molecules of soap and detergent form micelles in water. The hydrophobic part of soap dissolves in oil and grease while hydrophilic part of soap remains as free in soap solution. When the cloth is rubbed with hand or stirred mechanically, the big molecules of oil and soap break into small emulsified oil droplets.

These oil droplets repel each other due to the presence of anions of the hydrophilic part and do not precipitate. Thus they remain suspended in the soap solution without getting back on the cloth. These suspended oil and grease particles are then washed away by a stream of water.
Characteristics of Soaps:
- Soaps used for cleaning purpose are sodium or potassium salts of long chain fatty acids.
- They cannot be used in hard water or acidic solution as they precipitate out.
- They are biodegradable and hence they do not cause water pollution.
- e.g., stearic, oleic and palmitic acids.
Characteristics of Detergents:
- Detergents are sodium salts of long chain alkyl sulphates or a long chain of alkyl benzene sulphonate.
- Thay can be used in hard water and acidic medium.
- They are not biodegradable. Hence they cause water pollution.
- e.g. Sodium n-dodecyl benzene sulphonate, Cetyltrimethylammonium bromide, Pentaerythrityl stearate
Advantages of Detergents Over Soaps:
- Detergents can be used in hard water and acidic medium. While Soaps cannot be used in hard water or acidic solution as they precipitate out.
- Synthetic detergents have more solubility in water than soaps and hence they produce more lather.
- The cleansing action of detergents is stronger and efficient than soaps.