In this article, we shall study the coordination compounds, their terminology, and nomenclature
Double Salts:
These are the addition molecular compounds which are stable in the solid-state but dissociate into constituent ions in the solution. They are simply a combination of two different salts. e.g., Mohr’S salt, [FeSO4·(NH4)2SO4. 6H2O gets dissociated into Fe2+, NH+4, and SO2-4 ions.
Characteristics of Double Salts:
- They are simply a combination of two different salts. They are prepared by mixing the solution of two different salts in an equimolar ratio.
- They dissociate completely in their constituent ion in their solution and produce simple ions.
- They give a test of all individual species.
- They do not exhibit isomerism.
Complex Salts:
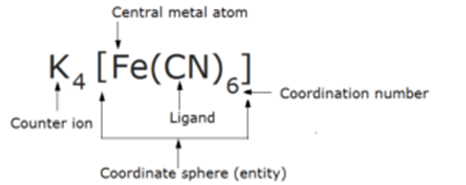
Complex salts are those molecular compound which contains a central metal atom coordinated with ligands within a coordination sphere. e.g., Potassium ferrocyanide, K4 [Fe(CN)6].
Characteristics of Complex Salts:
- They contain a central metal atom coordinated with ligands within a coordination sphere. It is prepared by mixing the solution of two different salts in a stoichiometric ratio.
- Complex salt does not dissociate completely into their constituent ions but produces complex-ions.
- They do not give a test of all individual species.
- They exhibit isomerism.
Coordination Compounds:
The transition metals form a large number of complex compounds in which the metal atoms are bound to a number of anions or neutral molecules, these compounds formed are called coordination compounds.
Definition: Coordination compounds are those addition molecular compounds which retain their identity in the solid-state as well as in the dissolved state.
In these compounds. the central metal atom or ion is linked by ions or molecules with coordinate bonds. e.g., Potassium ferrocyanide, K4 [Fe(CN)6].
Potassium ferrocyanide undergoes hydrolysis as
K4 [Fe(CN) 6]•3H2O → 4K+ + [Fe(CN) 6]4-
Coordination compounds play an important role in the chemical industry and in life itself. For example, the Ziegler-Natta catalyst which is used for polymerization of ethylene is a complex containing the metals aluminium and titanium. Chlorophyll, which is vital for photosynthesis in plants, is a magnesium complex and haemoglobin, which carries oxygen to animal cells, is an iron complex. Important vitamin B12 is a cobalt complex. Coordination compounds also find many applications in electroplating, textile dyeing and medicinal chemistry.
The complexes tend to retain their identity even in solution, although partial dissociation may occur. Complex-ion may be cationic, anionic or nonionic, depending on the sum of the charges of the central atom and the surrounding ions and molecules.
Terminology of Coordination Compounds:
Complex-ion or Coordination Entity or Coordination Sphere:
The central metal atom and the ligands which are directly attached to it are enclosed in a square bracket and are collectively termed as coordination sphere. The ligands and the metal atom inside the square brackets behave as single constituent unit
For e.g. in Potassium ferrocyanide, K4 [Fe(CN)6] the part [Fe(CN)6] is a coordination sphere. There are three types of coordination entities
- Cationic complex entity: It is the complex-ion which carries a positive charge. e.g., [Ni(NH3)6]2+ , [Pt(NH3)4]2+, etc.
- Anionic complex entity: It is the complex-ion which carries a negative charge. e.g., [PtCl4]2- , [Ag(CN)2]– , [Fe(CN)6]4- , etc.
- Neutral complex entity: It is the complex-ion which carries no charge. e.g., [Ni(CO)4] , [Co(NH3)4 Cl2]
Central Atom or Ion:
The atom or ion to which a fixed number of ions or groups are bound is called the central atom or ion. It is a Lewis acid. e.g., in Potassium ferrocyanide, K4 [Fe(CN)6], Fe is the central atom. The central atom is generally a transition element or inner-transition element.
Ligand:
The molecules or ions that are attached to the metal in a complex-ion are called ligands. A ligand is Lewis base. Every ligand has at least one unshared pair of valence electrons. The interaction between a metal atom and the ligands can be thought of as Lewis acid-base reaction. Some examples of Ligand are
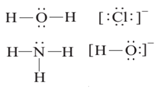
The atom in the ligand that is bound directly to the metal atom is known as the donor atom. In complex ion [Cu(NH3)4]2+, nitrogen is the donor atom while Cu2+ is the acceptor ion.
Depending on the number of the donor atoms present, ligands are classified as monodentate, bidentate or polydentate.
- Monodentate or Unidentate: It is a ligand, which has one donor site, e.g. H2O and NH3 are monodentate ligands
with only one donor atom in each. - Bidentate or Didentate: It is the ligand. which have two donor sites. ethylenediamine (en) is a bidentate ligand. The two nitrogen atoms can coordinate with a metal atom.

- Polydentate: It is the ligand, which has several donor sites. e.g., ethylenediaminetetraacetate ion. [EDTA]4- is a hexadentate ligand.

- Ambidentate Ligands: Some unidentate ligands have more than one donor atom and these may coordinate to the metal ion through either of the two atoms. These types of ligands are called ambidentate ligands. Complexes of these ligands yield linkage isomers.
M ← NO2 (N is the donor atom) or M ← ONO (O is the donor atom)
M ← SCN (S is the donor atom) or M ← NCS (N is the donor atom)
M ← CN (C is the donor atom) or M ← NC (N is the donor atom)
- Chelating (Greek: Chele, meaning “claw”) Ligands: Bidentate and polydentate ligands are also called chelating agents because of their ability to hold the metal atom like a claw. Stability of complex depends on the number of the chelating ring.
- Bridging Ligands: Some monodentate ligands can simultaneously coordinate two or more metal atoms. As a result, the ligand acts as a bridge between different metal atoms. It is, therefore, called a bridging ligand. The resulting Compound is known as a bridged complex.

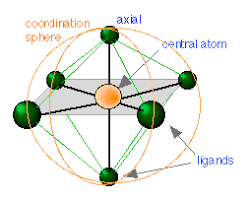
Coordination Polyhedron:
The spatial arrangement of the ligands which are directly attached to the central atom or ion is called coordination polyhedron around the central atom or ion.
Coordination number:
The coordination number in coordination compounds is defined as the number of ligands (donor) atoms/ ions surrounding the central metal atom in a complex ion.
| Complex ion | Ligand | Coordination number of |
| [Ni(NH3)6]2+ | (NH3) | Ni2+ is 6 |
| [Fe(CN)6]4+ | (CN) | Fe4+ is 6 |
| [Pt(NH3)4]2+ | (NH3) | Pt2+ is 4 |
| [PtCl4]2- | (Cl) | Pt2- is4 |
| [Ni(CO)4] | (CO) | Ni is 4 |
Oxidation number:
The charge of the complex if all the ligands are removed along with the electron pairs that are shared with the central atom, is called oxidation number of the central atom.
The net charge on a complex ion is the sum of the charges on the central atom and its surrounding ligands. In
[Fe(CN)6]4- , the net charge is – 4. Each cyanide group has the oxidation state of -1 so the oxidation number of Fe must be +2.
If the ligands do not bear net charges the oxidation number of the metal is equal to the charge of the complex ion. In
[Cu(NH3)4]2+ each NH3 is neutral, so the oxidation number of copper is +2.
IUPAC Nomenclature of Coordination Compounds:
Rules Of Nomenclature:
- The name of the compound is written in two parts (i) name of cation, and (ii) name of the anion.
- The cation is named first in both positively and negatively charged coordination complexes. For example, in K3[Fe(CN)6] name K+ first, and in [Co(NH3)4Cl2]Cl compound, name [Co(NH3)4Cl2] first.
- Within complex ligands (complex with dissimilar ligands) are named in alphabetical order first, and the metal
ion is named last. prefixes are ignored when alphabetizing ligands - For more than one similar ligands. the prefixes di, tri, tetra, etc are added before its name. For e.g. the ligands in the complex ion [Co(NH3)4Cl2]+ are named as “tetraamine dichloro”. If the di, tri, etc already appear in the complex then bis, tris, tetrakis are used. For e.g. the ligand ethylenediamine already contains di, therefore its name is bis(ethylenediamine).
- If the complex part is an anion, the name of the central metal ends with the suffix ‘ate’. Names of the anionic ligands end in ‘0’, names of positive ligands end with ‘ium’, and names of neutral ligands remain as such. But exceptions are we use aqua for H2O, ammine for NH3, carbonyl for CO, and nitrosyl for NO.
Naming of Central Atom
| Central Metal Atom | Name in Coordination Compound |
| Aluminum | Aluminate |
| Chromium | Chromate |
| Cobalt | Cobaltate |
| Copper | Cuperate |
| Gold | Aurate |
| Iron | Ferrate |
| Lead | Plumbate |
| Manganese | Manganate |
| Nickel | Nickelate |
| Rhodium | Rhodate |
| Silver | Argentate |
| Tin | Stannate |
| Zinc | Zincate |
Naming of Anionic Ligands
| Ligand | Name in Coordination Compound |
| Ammonia (NH3) | Ammine |
| Bromide (Br–) | Bromo |
| Carbon monoxide (CO) | Carbonyl |
| Carbonate (CO32-) | Carbonato |
| Chloride (Cl–) | Chloro |
| Cyanide (CN–) | Cyano |
| EDTA | Ethylenediamineteracetato |
| Ethylenediamine (NH2CH2CH2NH2) | Etylenediamine |
| Fluoride (F–) | Fluro |
| Hydroxide (OH–) | Hydroxo |
| Isothiocyanate (NCS– ) | Isothiocyanato |
| Oxide (O2–) | Oxo |
| Oxalate (C2O42-) | Oxalato |
| Sulphate (SO42-) | Sulphato |
| Thiocyanate (SCN–) | Thiocyanato |
| Water (H2O) | Aqua |
- The name of the complex part is written as one word.
- The oxidation state for the metal in cation, anion, or neutral coordination compounds is indicated by the Roman numeral in parentheses. For e.g. In complex cation [Cr(NH3)4Cl2]+, the oxidation number of chromium is +3 Hence the name of the ion is written as tetraamminedichlorochromium (III) ion. Similarly, complex anion [Fe(CN)6]4- is called hexacyanoferrate(II) ion.
- If the complex ion is a cation, the metal is named the same as the element. The neutral complex molecule is named similar to that of the complex cation. In complex cation [Cr(NH3)4Cl2]+ the name of ion is written as tetraamminedichlorochromium (III) ion, similarly cation [Co(NH3)6]3+ is named as hexaamminecobalt (III)ion. Neutral ion [Ni(CO) 4] is named as tetracarbonyl nickel(0),
Examples of IUPAC Naming:
| Coordination Compound | Type of Complex Ion | IUPAC Name |
| [Cr(NH3)3(H2O)3]Cl3 | Cation | triamminetrichlorochromium(III) chloride |
| [Co(H2NCH2CH2NH2)3]2(SO4)3 | Cation | tris (ethane-l,2-diamine) cobalt (III) sulphate |
| K4 [Fe(CN)6] | Anion | potassium hexacyanoferrate(II) |
| [Co(H2O)6]Cl3 | Cation | hexaaquacobalt(III) chloride |
| K2 [PtCl6] | Anion | potassium hexachloroplatinate(IV) |
| [Pt(NH3) 2Cl4] | Neutral | diamminetetrachloroplatinum(IV) |
| [Co(en) 2Cl2]Cl | Cation | dichlorobis(ethylenediamine)cobalt(III) chloride. |
| [Co(NH3) 4Cl2]+ | Cation | Tetraamminedichlorocobalt(III) |
| (NH4)3 [Cr(NCS) 6] | Anion | Ammonium hexathiocyanatochromate(III) |
| Ni(CO) 4 | Neutral | Nickel tetracarbonyl |
| [Cr(en) 3]Cl3 | Cation | Tris(ethylenediamine)cobalt(III) chloride |
| [Ag(NH3)2] [Ag(CN)2] | Neutral | diamminesilver (I)icyanoargentate(I) |