Generally, organic compounds are formed by covalent bonds among the constituent atoms. Carbon is tetravalent. It contains four electrons in its valence shell. One carbon can share its valence electrons with other elements or with another carbon to form the covalent bonds.
Methane is the simplest organic compound. One carbon atom shares its four electrons with four atoms of hydrogen forming four covalent bonds.
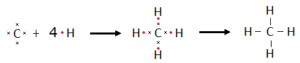
Modern Concept of Covalent Bonding:
Now it is assumed that the covalent bond between two atoms is formed due to overlapping of orbitals. The overlapping orbitals should be half filled and the spins of the electrons in the two overlapping orbitals should be opposite. The overlapping can take place by two ways viz. a) end to end overlapping or b) sidewise or parallel or lateral overlapping.
End to End Overlapping (Sigma Bond):
This type of bond is formed by overlapping of s-s, p-p and s-p orbitals. A sigma molecular orbital is formed by the end to end (or head-to-head) overlap of atomic orbitals along the internuclear axis. The overlap region is maximum in this bonding. The orbital is symmetrical to rotation about the internuclear axis. Sigma molecular orbital forms a strong bond called sigma(σ) bond.
Characteristics of Sigma Bond:
- It is a covalent bond formed by a coaxial overlap of bonding orbitals.
- It is a very strong bond, due to a greater extent of overlapping.
- It is possible between any two orbitals s-s, p-p or s-p and also hybrid orbitals.
- Bond energy is more.
Sidewise or Parallel or Lateral Overlapping (Pi Bond):
This type of bond is formed by overlapping of p-p orbitals. A covalent bond is formed by lateral or sideways or lateral overlapping of pure orbitals is known as pi bond.
The pi bond is formed by a lateral overlap of two p orbitals oriented mutually parallel but perpendicular to the internuclear axis.
Characteristics of the pi bond:
- Sigma bonds are stronger than pi bonds, hence pi bond can easily be broken than sigma bond.
- All the atoms directly attached to doubly bonded carbon atoms must be in the same plane.
- The p orbitals involved in pi bonds are parallel to each other and perpendicular to the internuclear axis and to the plane of the molecule.
- The rotation of the pi bond about the axis containing double bond interferes with the maximum overlap of p -orbitals. Hence such rotation is restricted. Thus molecule remains flat or planar.
- The electron cloud of overlapping orbital lie above and below the plane of the molecule. This allows the pi electrons available for attacking reagent.
Sigma Bond is Stronger Than a pi Bond:
- The extent of overlapping of orbitals along the same axes is always greater than the extent of overlapping at an angle. Also, the larger the overlapping of orbitals, the stronger is the covalent bond.
- A sigma bond is formed by the co-axial overlapping of atomic orbitals. A pi bond is formed by lateral or sidewise overlapping of atomic orbitals.
- Due to the large overlap of atomic orbitals, the sigma bond involves more evolution of energy than the Pi bond. In a sigma bond, the electron density between two nuclei on the internuclear axis is very high. In the case of a pi bond, the electron density is higher above and below the internuclear axis and not on the nuclear axis.
- Hence the sigma bond in which overlapping of orbitals takes place along the same axis is stronger than the pi bond in which overlapping of orbitals takes place at an angle (literally).
Concept of Hybridization in Carbon:
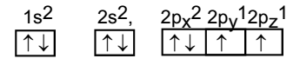
The atom of Carbon C (Z = 6) Electronic configuration is 1s2 2s2 2p2 (With two unpaired electrons).
According to valence bond theory valency of an element depends on a number of unpaired electrons in the orbitals. Thus carbon should be divalent. However, the valency of carbon is 4. Valence bond theory fails to explain this change of valency.
The simplest molecule is that of methane. The methane molecule possesses following characteristics.
- All C-H bonds are identical
- All the bonds do not lie in the same plane.
- The H-C-H bond angle is found to be 109° 28′.
This change in the valency and equivalency of the 4 C-H bonds is explained on the basis of hybridization theory.
Mixing and recasting or orbitals of an atom(same atom) with nearly equal energy to form new equivalent orbitals with maximum symmetry and definite orientation in space is called as hybridization.
In carbon three types of hybridization viz. sp3, sp2 and sp are possible.
SP3 Hybridization (Formation of Methane Molecule):
Step -1: Formation of the excited state of a Carbon atom:
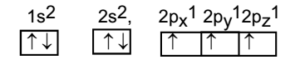
The carbon atom in the ground state takes up some energy and goes to the excited state. In this process, a pair of electrons in 2s orbital splits up and one of the electrons from this pair is transferred to an empty 2pz orbital. Thus the excited state has four half-filled orbitals.
Ground Stae Configuration
Excited Stae Configuration
Step – 2: Hybridization of Orbitals:
One orbital of 2s and three orbitals of 2p mix up forming four hybrid orbitals of equivalent energy. These four new equivalent orbitals are called sp3 hybrid orbitals. They are identical in all respect.


Angle and Geometry:
- Four sp3 hybridized orbitals formed, repel each other and they are directed towards the four corners of a regular tetrahedron. The Angle between them is 109.5°.
- Each sp3 hybrid orbital contains one unpaired electron.
- In each sp3 hybrid orbital, one of the lobes is bigger because of more concentration of electron density. Only bigger lobe is involved in bond formation. Due to this maximum overlapping is achieved.
- Four sp3 hybrid orbitals of carbon atom having one unpaired electron each overlap separately with 1s orbitals of four hydrogen atom along the axis forming four covalent bonds
- Thus in CH4 molecule has a tetrahedral structure with a carbon atom at the centre and four hydrogens at the four corners of a regular tetrahedron. H-C-H bond angle is 109.5°.
Bonds:

- Four sp3 hybrid orbitals of carbon atom having one unpaired electron each overlap separately with 1s orbitals of four hydrogen atom along the axis forming four covalent bonds (sigma bonds).
- The bonds between carbon and hydrogen are sp3– s.
- Thus H – C — H bond angles are 109.5°. The molecule is tetrahedral.
- All C-H bond in methane are of equal strength.
Type and Geometry of Methane Molecule:
| Name of Molecule | Methane |
| Molecular Formula | CH4 |
| Type Of Hybridisation | sp3 |
| Geometry | Tetrahedral |
| No. Of Bonds | 4 |
| No. Of Sigma bonds | 4 sigma |
| Bond angle | 109.5° |
| Overlaps | 4 sp3 – s |
| Bonds | 4 C-H |
sp2 Hybridization (Formation of Ethylene Molecule):
In ethylene, there is sp2 hybridisation of carbon atom. One 2s orbital and two 2p orbitals of carbon mix up forming three hybrid orbitals of equivalent energy. These three new equivalent orbitals are called sp2 hybrid orbitals. They are identical in all respect. One ‘p’ orbital of each carbon atom remains unhybridised.
Step -1: Formation of the excited state of a Carbon atom:
The carbon atom in the ground state takes up some energy and goes to the excited state. In this process, a pair of electrons in 2s orbital splits up and one of the electron from this pair is transferred to empty 2pz orbital. Thus the excited state has four half filled orbitals.
Step – 2: Hybridization of Orbitals:
One orbital of 2s and two orbitals of 2p mix up forming three hybrid orbitals of equivalent energy, while the third p orbital remains unhybridized. The three new equivalent orbitals are called sp2 hybrid orbitals. They are identical in all respect.


Angle and Geometry:
Three sp2 hybridised orbitals formed, repel each other and they are directed in a plane towards the three corners of an equilateral triangle. The angle between them is 120°. The unhybridised pz orbital remains perpendicular to this plane. Each sp2 hybrid orbital contains an unpaired electron. In each sp2 hybrid orbital, one of the lobes is bigger because of more concentration of electron density. Only bigger lobe is involved in bond formation.
As all the six atoms in the C2H4 molecule being in the same plane, the molecule is planar. H-C-H bond angle is 120°. H-C-C bond angle is 120°.
Bond:
Sigma Bond Formation:
- One sp2 hybrid orbital of one carbon atom overlaps with One sp2 hybrid orbital of another carbon atom head-on forming a sigma bond. One (sp2– sp2 ) – σ bond
- Remaining two hybrid orbitals of each carbon atom overlap with ‘s’ orbital of four hydrogen atoms separately forming four sigma bonds. (4 C–H). Four (sp2– s) – σ bond. All C-H bonds in ethylene are of equal strength. Thus there are five sigma bonds. Sigma bonds are stronger.
Formation of pi Bond:
The unhybridised 2 pz orbitals of each carbon atom being perpendicular to the plane of four hydrogen atoms and carbon atoms overlap laterally with one another to form a week pi bond between two carbon atoms by p-p overlap. One (p-p) – π bond.This bond consists of two equal electron cloud one lying above the plane of the atom and other lying below this plane.
Thus in ethylene molecule, there are 5 sigma bonds and 1 pi bond.
Diagram:

Type and Geometry of Ethylene Molecule:
| Name of Molecule | Ethylene |
| Molecular Formula | C2H4 |
| Type Of Hybridisation | sp2 |
| Geometry | Trigonal planar |
| No. Of Bonds | 6 |
| No. Of Sigma bonds | 5 sigma |
| No. of pi Bonds | 1 |
| Overlaps | One (sp2– sp2 ) – σ bond Four (sp2– s) – σ bond One (p-p) – π bond |
| Bond angle | H-C-C 120° and H-C-H 120° |
| Overlaps | 4 sp2 – s, 1 sp2– sp2 |
| Bonds | 4 C-H Single Bond ( 4 sigma) 1 C-C Double bond ( 1 sigma 1 pi) |
sp Hybridization (Formation of Acetylene Molecule):
In acetylene, there is sp hybridisation of carbon atom. One 2s orbital and one 2p orbital of carbon mix up forming two hybrid orbitals of equivalent energy. These two new equivalent orbitals are called sp hybrid orbitals. They are identical in all respect. Twp ‘p’ orbitals of each carbon atom remain unhybridised.
Step -1: Formation of the excited state of a Carbon atom:
The carbon atom in the ground state takes up some energy and goes to the excited state. In this process, a pair of electrons in 2s orbital splits up and one of the electron from this pair is transferred to empty 2pz orbital. Thus the excited state has four half filled orbitals.
Step – 2: Hybridization of Orbitals:
One 2s orbital and one 2p orbital of carbon mix up forming two hybrid orbitals of equivalent energy. These two new equivalent orbitals are called sp hybrid orbitals. They are identical in all respect. Two ‘p’ orbitals of each carbon atom remain unhybridized.


Angle and Geometry:
- Two sp hybridized orbitals formed, repel each other and These hybrid orbitals are arranged along the x-axis in a linear manner around central carbon atom and are at an angle of 1800 to one another.
- The unhybridized py and pz orbital remain perpendicular to hybrid orbitals along the y-axis and z-axis, mutually perpendicular.
- Each sp hybrid orbital and un-hybrid orbitals contain an unpaired electron.
- In each sp hybrid orbital, one of the lobes is bigger because of more concentration of electron density. The only bigger lobe is involved in bond formation.
- As all the four atoms in the C2H2 molecule being in the same line, the molecule is diagonal or linear. H-C-C bond angle is 180°.
Bond:
Sigma Bond Formation :
- One sp hybrid orbital of one carbon atom overlaps with One sp hybrid orbital another carbon atom head on forming a sigma bond. One (sp- Sp ) – σ bond
- Remaining one hybrid orbital of each carbon atom overlap with ‘s’ orbital of two hydrogen atoms separately forming two sigma bonds. (2 C–H). Two (sp– s) – σ bond. Both C-H bonds in Acetylene are of equal strength. Thus there are Three sigma bonds. Sigma bonds are stronger.
Formation of pi Bon
- The unhybridized 2 py and 2 pz orbitals of each carbon atom being perpendicular to each other and to the plane of H-C-C-H axis overlap laterally with one another to form two week pi bond between two carbon atoms by two p – p overlap. (one 2 py -2 py )and (one 2pz-2 pz) . Thus two (p-p) – π bond.
- In the ethylene molecule, there are 3 sigma bonds and 2 pi bonds. There is a Tripple bond between carbon and carbon consisting of one sigma and two pi bonds.
Diagram

Type and Geometry of Acetylene Molecule :
| Name of Molecule | Acetylene |
| Molecular Formula | C2H2 |
| Type Of Hybridisation | sp |
| Geometry | Diagonal or Linear |
| No. Of Bonds | 5 |
| No. Of Sigma bonds | 3 |
| No. of pi Bonds | 2 |
| Overlaps | One (sp- sp ) – σ bond Two (sp– s) – σ bond Two (p-p) – π bond |
| Bond angle | H-C-C 180° |
| Bonds | C-H Single Bond ( 2 sigma) C-C Tripple bond ( 1 sigma and 2 pi) |
How to Predict Type of Hybridization of Carbon:
- A carbon atom that is directly attached to four other atoms is sp2 hybridized.
- A carbon atom that is directly attached to three other atoms is sp2 hybridized.
- A carbon atom that is directly attached to two other atoms is sp hybridized.
Example – 1: To find hybridization state of each carbon atom in the following molecule.
CH3CHO (Acetaldehyde):

Carbon 1 is attached to three other atoms. Hence it is sp2 hybridized.
Carbon 2 is attached to four other atoms. Hence it is sp3 hybridized.
C6H6 (Benzene):

Each carbon is attached to three other atoms. Hence it is sp2 hybridized.
CH2=C=CH2 (Propadiene):

Carbon 1 and 3 are attached to three other atoms. Hence it is sp2 hybridized.
Carbon 2 is attached to two other atoms. Hence it is sp hybridized.
CH3-CO-CH3 (Acetone):

Carbon 1 and 3 are attached to four other atoms. Hence it is sp3 hybridized.
Carbon 2 attached to three other atoms. Hence it is sp2 hybridized.
Complex Molecules:

Carbon 2 and 6 are attached to four other atoms. Hence it is sp3 hybridized.
Carbon 1, 3, 4, 5 and 7 are attached to three other atoms. Hence it is sp2 hybridized.
How to predict the shape of a molecule.
HCHO (Formaldehyde):
Carbon in formaldehyde is attached to three other atoms. Hence it is sp2 hybridized. Due to sp2 hybridization of carbon, formaldehyde has a planar structure.
CH3NO2 (Nitromethane):
Carbon in nitromethane is attached to four other atoms. Hence it is sp3 hybridized. Due to sp3 hybridization of carbon, nitromethane has a tetrahedral structure.
HC≡N (Hydrogen cyanide):
Carbon in hydrogen cyanide is attached to two other atoms. Hence it is sp hybridized. Due to sp hybridization of carbon, hydrogen cyanide has a linear structure.
Calculation of the number of σ and π bonds:
Notes:
- All single bonds are sigma bonds.
- All double bonds consist of one sigma bond and one pi bond.
- All triple bonds consist of one sigma bond and two pi bonds.
Find the number of sigma and pi bonds in the following molecules.
Steps to Solve Question:
- Draw the dash structure of the molecule
- Calculate the number of single bonds, double bonds, and triple bonds.
- The number of Sigma bonds = Number of single bonds + Number of double bonds (if any) + Number of triple bonds (if any). If the double bond and triple bond are not present do not consider them for calculation
- The number of pi bond = Number of double bonds + 2 x Number of triple bonds (if any). If triple bonds are not present do not consider them for calculation.
CH3-CH=CH2 (Propene):
There are seven single bonds and 1 double bond. Hence number of sigma bond = 7 + 1 = 8 and number of pi bonds is 1.
CH3-CH2-CH2-CH3 (Butane):
There are 13 single bonds. Number of sigma bond = 13
CH3-C≡C-CH3 (But-2-yne):
There are eight single bonds and 1 triple bond. Hence number of sigma bond = 8 + 1 = 9 and number of pi bonds is 2.
CH3-C≡C-H (Propyne):
There are 5 single bonds and 1 triple bond. Hence number of sigma bond = 5 + 1 = 6 and number of pi bonds is 2
CH2Cl2 (Dichloromethane):
There are 4 single bonds. Number of sigma bond = 4.
CH2=C=CH2 (Propadiene):
There are 4 single bonds and 2 double bonds. Hence number of sigma bond = 4 + 2 = 6 and number of pi bonds is 2.
C6H6 (Benzene):

There are 9 single bonds and 3 double bonds. Hence number of sigma bond = 9 + 3 = 12 and number of pi bonds is 3.
C6H12 (Cyclohexane):

There are 18 single bonds. Number of sigma bond = 18
One reply on “Bonding in Organic Compounds”
This is chemistry made easy. Excellent explanations of basic concepts to the understanding of all. Keep up!