Science > Chemistry > Third Row Elements > Molecular Solids of the Third Row
in this article, we shall study the crystal structure of molecular solids of third perid of periodic table.
Molecular solid:
The substance in which lattice points are molecules which are held together by means of weak physical forces (van der Waal’s forces) are called molecular solid. Phosphorous, sulphur, chlorine, and argon are molecular solids because lattice points are molecules.
They have greater ionization potential and vacant valency orbitals are not available.
Characteristics of Molecular Solids:
- In the crystal structure of these elements, the units occupying lattice points are molecules
- They have a greater ionization potential
- The molecules are attached to each other by weak van der Wall’s forces of attraction.
- In these solids, the atoms are joined together within the molecule by strong covalent bonds.
- In these solids, vacant valency orbitals are not available. All the valence orbitals are used for intra-molecular strong covalent bonding.
Scientific Reasons:
Phosphorous, Sulphur, Chlorine, and Argon as solid are soft easily compressible and distorted. They are volatile their boiling points and melting points are very low.
In the crystal structure of these elements, the units occupying lattice points are molecules. They have a greater ionization potential. The molecules are attached to each other by weak van der Wall’s forces of attraction. Within the molecules, the atoms are joined together by strong covalent bonds. Thus they are soft easily compressible and distorted.
Similarly less energy is required to separate the molecules from each other in a molecular crystal. Hence they are volatile and possess low boiling points and melting points.
Phosphorous and Sulphur are solids at room temperature while Chlorine and Argon are gases at room temperature.
In the crystal structure of these elements, the units occupying lattice points are molecules. They have a greater ionization potential. The molecules are attached to each other by weak van der Wall’s forces of attraction. Within the molecules, the atoms are joined together by strong covalent bonds.
Sulphur is Octaatomic, Phosphorous is tetra atomic, Chlorine is diatomic, Argon is monoatomic. The size of the molecule decreases in the order S8; P4 > Cl2 > Ar. van der Wall’s forces of attraction decrease in the same order. They are stronger in phosphorous and sulphur while negligible in chlorine and argon. Thus Chlorine and Argon have very low boiling and melting points. Hence Phosphorous and Sulphur are solids at room temperature while Chlorine and Argon are gases at room temperature.
Structure of Molecular Solids:
Structure of Phosphorous:
Phosphorous is molecular solid because lattice points are P4 molecules which are held together by means of van der Wall’s forces of attraction. Within the molecules, the atoms are joined together by strong covalent bonds.
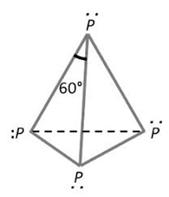
The atomic number of Phosphorous is 15. Electronic configuration of phosphorous is, 1s2, 2s22p6, 3s2 , 3px1 3py1 3pz1. Phosphorous has greater ionization potential. Due to the non-availability of vacant valency orbitals, it is a molecular solid. Phosphorous atoms are sp3 hybridized.
One phosphorous atom forms three covalent bonds with three other phosphorous atoms while one sp3 hybrid orbital contained paired electrons (lone pair) remain non-bonded. Thus tetrahedral P4 molecule is formed. The P-P-P bond angle is 60°. The phosphorous molecule consists of four phosphorous atoms in it.
In white phosphorous, these tetrahedral molecules are joined together by means of weak van der Waal’s forces of attraction due to smaller molecular size. So white phosphorous has a low melting point.
Red phosphorous is a polymeric form. In red phosphorous one P-P bond of P4 unit gets ruptured and freed bonds link up to form a chain. Thus in red phosphorous, the tetrahedral molecules are joined to one another by means of strong covalent bonds, forming a chain like structure. Hence red phosphorous exists in polymeric form.
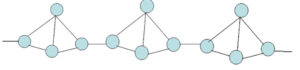
In white phosphorous, the molecules are joined together by weak van der Wall’s forces while in red phosphorous molecules are bound by strong covalent bonds. Therefore more energy is required for breaking bonds between molecules of red phosphorous than that in white phosphorous. Hence red phosphorous shows a high melting point and less reactivity than white phosphorous.
Structure of Sulphur:
Sulphur is molecular solid because lattice points are S8 molecules which are held together by means of van der Waal’s forces of attraction. Within the molecules, the atoms are joined together by strong covalent bonds.
The atomic number of Sulphur is 16. Electronic configuration of phosphorous is 1s2, 2s22p6, 3s2 , 3px2 3py1 3pz1. Sulphur has greater ionization potential. Its two half-filled orbitals are used for intermolecular bonding. Due to the non-availability of vacant valency orbitals, it is a molecular solid. Each Sulphur molecule consists of eight sulphur atoms in it.

The sulphur molecule has a puckered ring structure or crown structure. Sulphur has two half-filled 3p orbitals in the valency shell. Within each S8 molecule, each s atom is linked to two adjacent S atoms by the covalent single bond. Thus if seen from the top it forms a ring-like structure with four sulphur atoms in one plane and alternate other four in a parallel plane. Each Sulphur atom has a lone pair of electrons. The S-S-S bond angle is 107.8 0 and S-S bond length is 2.04 o
Structure of Chlorine:
Chlorine is molecular solid because lattice points are Cl2 molecules which are held together by means of van der Waal’s forces of attraction. Within the molecules, the atoms are joined together by strong covalent bonds.
The atomic number of Chlorine is 17. Electronic configuration of phosphorous is 1s2, 2s22p6, 3s2 , 3px2 3py2 3pz1. Chlorine has greater ionization potential. Hence it forms covalent bonding. Its one half-filled orbital is used for intermolecular bonding. Due to the non-availability of vacant valency orbitals, it is a molecular solid. Each chlorine molecule consists of two Chlorine atoms in it bonded by a covalent bond.
Chlorine in solid state consists of layers of chlorine molecules which are held by weak Wander Wall’s forces. Hence Chlorine is gas at room temperature.
Structure of Argon:
The substance in which lattice points are molecules which are held together by means of weak physical forces (vander waal’s forces) are called molecular solids.
Argon is a molecular solid or atomic solid because lattice points are Ar atoms (molecules) which are held together by means of van der Waal’s forces of attraction. The atomic number of Argon is 18. Electronic configuration of Argon is 1s2, 2s22p6, 3s2 , 3px2 3py2 3pz1. Thus its octet is complete. It has no unpaired electrons. Argon has greater ionization potential. Due to the non-availability of vacant valency orbitals, it is a molecular solid. Each Argon molecule consists of one Argon atom.
Argon crystal in its solid-state consists of a continuous pattern of atoms giving rise to a face centred closed pack cubic lattice like aluminium. But the difference is that in the case of aluminium lattice points are aluminium ions while in case of argon lattice points are Argon atoms. Melting and boiling points of argon are very low.