Science > Chemistry > Third Row Elements > Metallic solids of the Third Row
In this article, we shall study properties metallic solids of third row.
The metallic bond is defined as the force of attraction that binds metal cations to a number of mobile or delocalized electrons within its sphere of influence which holds the metallic cations together in a definite pattern. On the basis of metallic bonding and type of crystal structure, the general properties of metals like high melting point, thermal conductivity, electrical conductivity, malleability, and ductility can be explained.
Characteristics of Metallic Solids:
- They possess high melting and boiling points
- Thy show high thermal conductivity
- They show high electrical conductivity
- They are malleable and ductile
Reasons for High Melting and Boiling Points of Metallic Solids:
- Metals possess a very strong metallic bond.
- In order to break a strong metallic bond more heat is required.
- Due to strong metallic bonding and closely packed crystal lattices, metals have higher melting and boiling points.
- Metallic bond strength depends on the quantity of charge carried by metal ions in the metallic bonding and hence the melting and boiling points of Na, Mg and Al are in the order. Al > Mg > Na.
- Among Na, Mg, and Al, aluminium has greater melting and boiling point due to a very strong metallic bond and closely packed crystal lattice.
Reasons for High Thermal Conductivity of Metallic Solids:
In metals, cations are surrounded by many mobile electrons. The loosely bound delocalized electrons can move freely in a crystal lattice. When one end of the metal is heated, delocalized electrons acquires the heat energy, their Kinetic energy increases. The mobility of these delocalized electrons increases. They convey heat moving rapidly throughout the crystal lattice from the hot end to the cooler end. During this process, they collide with adjacent electrons and thus transfer part of their energy to adjacent electrons. Thus heat is transferred from one end to other due to mobile electrons and collisions.
Thus due to the presence of free, mobile electrons, metals have high thermal conductivity. Thermal conductivity depends on the number of mobile electrons. Thus the thermal conductivity increases from Na to Al as the number of valence electrons increases. Thermal conductivity increases in the order Na < Mg < Al
Reasons for High Electrical Conductivity of Metallic Solids:
In metals, cations are surrounded by many mobile electrons. The loosely bound delocalised electrons can move freely in a crystal lattice. The delocalised electrons in metals account for the electrical conductivity.
When an electric field i.e. potential difference is applied across the two ends of a metallic wire, the free electrons are displaced from the negative end to the positive end and conducts electricity. Thus high electrical conductivity of metals is due to the presence of mobile valence electrons.
Electrical conductivity depends on the number of mobile electrons. Thus the electrical conductivity increases from Na to Al as the number of valence electrons increases. Electrical conductivity increases in the order Na < Mg < Al
Malleability and Ductility:
The property of metals by which they can be flattened into thin shits by hammering is known as malleability. The property of metals by which they can be drawn out into wires by stretching is known as ductility. Malleability takes places under compression and ductility takes place under tension.
In metals, cations are surrounded by many mobile electrons. The loosely bound delocalised electrons can move freely in a crystal lattice. The metallic bond in the metal crystal is non-directional and not rigid because each metal ion is surrounded by many mobile electrons. The mutual repulsion between metal ions is neutralized by the sea of electrons that moves around them.
When stress is applied to the metal surface, one layer of metal ions slides over the other. This relative movement of layers with respect to each other is called slip. After slipping the ions settle into new positions keeping the distance between the metal ions same. At the same time, the mobile electrons also move along the metal ions. The crystal structure of the metal, therefore, does not change but the shape of the metal changes.
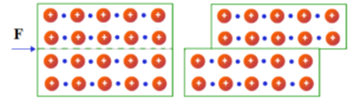
Thus a metal can be flattened into sheets without breaking of any strong metallic bonds and any change in its internal structure. The same thing happens when metal is drawn into wire.
Malleability and ductility of metal depend upon the crystal structure. Amongst Na, Mg, and Al, aluminium is more malleable and ductile because aluminium has more closely packed face centred cubic structure in which empty space is just about 26% of the available space in the unit cell and has a strong metallic bond. The order of malleability or ductility is Na < Mg < Al
Metallic lustre:
The clean surface of nearly all metals have shinning surfaces and reflects light giving the metals a silvery colour. i.e. lustre. In metals, cations are surrounded by many mobile electrons. The loosely bound delocalized electrons can move freely in a crystal lattice. Metallic lustre is due to the presence of free mobile electrons in the crystal lattice of metals.
When a beam of light falls on the surface of the metal, the mobile electrons present in the surface absorb the photons of incident light and get excited to the higher energy state. But this absorbed light energy is immediately re-emitted in the form of electromagnetic radiations of same frequencies by returning to original energy level. Consequently, due to this momentary exchange of light energy, the metal surface exhibit shinning appearance.
Na, Mg, and Al of the third row show bright lustre when freshly cut, because being chemically active, the surface of these metals gets coated with oxide, hydroxide, carbonate etc. formed due to their interaction with the atmospheric gases.
Electrical Conductivity of Silicon:
Non-Conductivity at absolute zero:
The atomic number of Silicon is 14. Electronic configuration of silicon in its ground state is 1s2, 2s22p6, 3s2 3p2. It has four valence electrons in their valence orbitals. Silicon undergoes sp3 hybridisation forming four sp3 hybridised orbitals of equal energy.
Each silicon atom forms four covalent bonds with four other neighbouring silicon atoms due to SP3– SP3 Thus tetrahedral Si4 unit is formed which is extended to form a three-dimensional giant molecule. Due to overlapping of hybrid orbitals, Si-Si covalent bonds are very strong and are directional. In each bond, there are 2 electrons one contributed by each atom.
Thus all valence electrons are used in the formation of covalent bonds. Thus the electrons are localised. Due to which no free electrons are available for conduction. Thus Silicon is an insulator at absolute zero.
Intrinsic Conductivity of Silicon:
Due to heat or at room temperature, some of the covalent bonds in the crystal lattice break up and release free electrons. Thus free electrons are available for conduction of electricity. When a potential difference is applied across the crystal these free electrons move towards the positive terminal of battery leaving behind a hole in the bond.
As the temperature increases the electron-hole pairs formed increase. Electrons move to positive pole and hole moves towards the negative pole of the battery. This conduction of electric current by pure semiconductor at high temperature is called intrinsic conductivity.
On lowering the temperature freed electrons fall back to their position in the hole. Thus due to less availability or unavailability of free electrons, electrical conductivity decreases.
Note:
Due to overlapping of hybrid orbitals in silicon, Si-Si covalent bonds are very strong and are directional. Presence of network of strong covalent bonds accounts for the hardness and its high melting point. Similarly it shows no malleability and no ductility.