Science > Chemistry > Third Row Elements > Concept of Metallic Bond and Metallic Solids
In this article, we shall study the concept of metallic bond and metallic bonds in sodium, magnesium and aluminium crystals.
Concept of Metallic Bond:
The metallic bond is defined as the force of attraction that binds metal cations to a number of mobile or delocalized electrons within its sphere of influence which holds the metallic cations together in a definite pattern. To explain the nature of metallic bond many theories were proposed. Free electron theory or electron sea theory is one of the simplest theory proposed by Drude and Lorentz. Some of the important postulates of this theory are as follows:
Free Electron Theory of Metallic Bond:
Metal atoms have less number of valence electrons so they have many vacant valence orbitals. Na, Mg and Al have 3p orbitals vacant.
| Name | Symbol | Atomic No. | Electronic configuration | Detailed configuration |
| Sodium | Na | 11 | 2, 8, 1 | 1s2, 2s2 2p6, 3s1 |
| Magnesium | Mg | 12 | 2 , 8, 2 | 1s2, 2s2 2p6, 3s2 |
| Aluminium | Al | 13 | 2, 8, 3 | 1s2, 2s2 2p6, 3s2 3p1 |
Ionization potential values of metals are low, hence valence electrons are loosely held and can be easily removed. The closely packed structure of the metallic crystal consists of the atoms of the metal which are identical in all respects.
The unoccupied orbitals of closely packed atoms of the metal overlap with the similar orbital of adjacent atoms through the crystal lattice. Valence electrons removed from their orbitals and can move freely from vacant valency orbits of one atom to other. Since these valence electrons do not belong to any single atom but to crystal as a whole, they are called delocalized or mobile electrons. The metal ions (cations) produced due to delocalization are called kernels. The metal ions have fixed positions in the crystal lattice while delocalized electrons are free to move in the crystal lattice. Thus metal can be considered as an aggregation of metal cations immersed in a sea of mobile electrons.
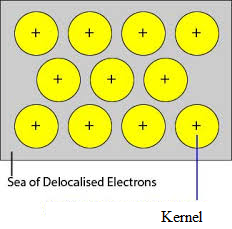
Since the electrons in the metals are delocalized, and they are assumed to be uniformly distributed throughout the crystal lattice. The forces of attraction between the metal ions and delocalized electrons are uniform in all the direction. Hence the metallic bond is non-directional.
The units occupying lattice points in Sodium, Magnesium, Aluminium are positive ions of them respectively and are surrounded by mobile electrons. Thus Sodium, Magnesium, and Aluminium are metallic solids.
As we move from left to right i.e. from Sodium to Aluminium the no. of valence electrons increases and hence the strength of the bond increases from Sodium to Aluminium.
The properties like electrical and thermal conductivity, metallic lustre, malleability and ductility can be explained on the basis of free electron theory.
Characteristics of Metallic Bonds:
- The metallic bond is defined as the force of attraction that binds metal cations to a number of mobile or delocalized electrons within its sphere of influence which holds the metallic cations together in a definite pattern.
- The metallic bond is non-directional.
- They are weaker than the covalent bond but stronger than van der Waal’s forces.
- The bonds are not rigid.
- The strength of the metallic bond is directly related to the positive charge on the metal ion. So the strength of metallic bond increases as Na < Mg < Al.
Metallic Solids:
Metallic solids are crystalline solids in which the units occupying lattice points are positive ions surrounded by a pool of electrons. (Concept of metallic bond)
Crystal Structures of Metals:
X-ray analysis of different metallic crystals have shown that metals adopt either of the following crystal structures.
- Body centred cubic structure. (BCC)
- Face centred cubic structure. (FCC)
- Hexagonal close-packed structure. (HCP)
Sodium (Na):
Sodium metal has body centred cubic (BCC) open packed crystal structure. Bonding is non-directional metallic bonding.
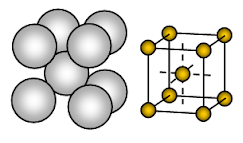
The arrangement of ions in one plane- Cubic array – open or square packed structure. In this arrangement, each metal ion is touching four adjacent ions in one plane. The sequence of the layers is AB, AB, AB, ……….
Sodium is a metallic solid. In the unit cell of sodium, each sodium ion is surrounded by eight other equidistant sodium ions. Hence co-ordination number is 8. These sodium ions are arranged at the corners of an imaginary cube and at the centre of the cube, one sodium ion is present. There are 2 ions present in one unit cell of sodium.
The sodium ions occupy only about 68% of the available space in a unit cell. So 32% of the unit cell remains empty (void). Since this structure has more empty space metals which adopt this structure are soft. Sodium is thus soft metal because of more empty space (about 32% ) in its crystal structure and rather weak metallic bond due to just one valence electron per Na atom in its crystal.
Magnesium (Mg):
Magnesium has hexagonal close-packed (HCP) crystal structure. Bonding is non-directional metallic bonding.
Magnesium is metallic solid. The units occupying lattice sites are Mg ions and these ions are surrounded by mobile or delocalized electrons.
The arrangement of ions in one plane the arrangement of ions is a hexagonal array or closed packed layer. Thus each metal ion is touching six adjacent ions in one plane. Each magnesium ion touches six magnesium ions in its own layer, three in the layer above and three in the layer below. In hexagonal packed structure, the closed packed layers of ions are stacked in an alternating sequence usually called AB ABA ….. Every third layer of ions is exactly the same as and lie directly above the first layer.
Each Mg atom is surrounded by 12 other equidistant Mg ions. Hence co-ordination number is 12. In the unit cell, about 26% of the available space is empty (void). This structure is more closely packed. Due to less empty space in the crystal structure, more electron cloud due to Mg+2 and strong metallic bond, magnesium is harder than sodium metal. It is more malleable and ductile than sodium.
Aluminium (Al):
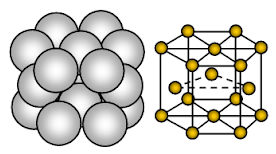
Aluminium has face centred cubic (FCC) crystal structure. Bonding is non-directional metallic bonding.

Aluminium is metallic solid. The units occupying lattice sites are Al ions and these ions are surrounded by mobile or delocalised electrons.
The arrangement of ions in one plane the arrangement of ions is a hexagonal array or closed packed layer. Thus each metal ion is touching six adjacent ions in one plane.
In a cubic close-packed or face centred cubic crystal structure, the sequence of close-packed layers of ion repeats every fourth layer. I.e. every fourth layer of ions is exactly the same as and lies directly above the first layer. It is thus called ABC, ABC, ABC, ….. cubic close packing. Cubic close packing is also called as face centred close-packed structure because if viewed from a particular angle, the ions can be considered as being at the eight corners of the cube and at the centre of each of the six faces of the cube i.e. unit cell.
Each aluminium ion (Al+3) is surrounded by 12 other equidistant aluminium atoms. Hence co-ordination number is 12. In the unit cell of aluminium, about 26% of the available space is empty (void). Due to less empty space in the crystal structure, strong metallic bonding, aluminium is harder, more malleable and ductile than sodium and magnesium.
Silicon (Si):
Covalent solids are crystalline solids in which unit lattice points are atoms. The major binding force is covalent bonds between atoms. Silicon is a covalent solid in which lattice points are occupied by the atoms of the element. Silicon is a network solid. There is a network of Si-Si covalent bonds.
The atomic number of Silicon is 14. Electronic configuration of silicon in its ground state is 1s2, 2s22p6, 3s2 3p2. It has four valence electrons in their valence orbitals. Silicon undergoes sp3 hybridisation forming four sp3 hybridised orbitals of equal energy. Each silicon atom forms four covalent bonds with four other neighbouring silicon atoms due to SP3– SP3 Thus tetrahedral Si4 unit is formed which is extended to form a three-dimensional giant molecule.

The Si -Si bonds run continuously throughout the crystal. Thus a crystal of silicon is regarded as a giant three-dimensional molecule having a tetrahedral network of silicon atoms bonded together by strong covalent bonds. Solid containing such a structure is called network solid. Si-Si bond angle is 109 o 28 ’ while the bond length is 2.35 o
Due to overlapping of hybrid orbitals, Si-Si covalent bonds are very strong and are directional. Presence of network of strong covalent bonds accounts for the hardness and its high melting point.
The electrons in the covalent bond are localized hence silicon is not a good conductor of electricity and heat. But it is a semiconductor. Due to the non-availability of free electrons, silicon is an insulator at absolute zero temperature. However, silicon is a semiconductor. If the temperature is increased, covalent bonds break electrons set free which can conduct electricity. Thus conductivity increases with increase in temperature.