Science > Chemistry > Third Row Elements > Oxidizing and Reducing Property of the Third Row Elements
In this article, we shall discuss the trend in oxidizing and reducing property of third row elements.
Oxidation:
The process in which an atom, a molecule or an ion loses one or more electrons is called oxidation. It is also known as de-electronation.
e.g. Na → Na+ + e–
In this case, the oxidation of sodium is taking place.
Reduction:
The process in which an atom, a molecule or an ion gains one or more electrons is called reduction. It is also known as electronation.
e.g. CI + e– → Cl–
In this case, the reduction of chlorine is taking place.
Reducing Agent:
A substance (an atom, a molecule or an ion) which forces another substance to accept electrons and it itself undergoes oxidation by losing electrons is called the reducing agent. Reducing agent is electron donor. e.g. Na, Al, Mg etc.
Oxidizing Agent:
A substance (an atom, a molecule or an ion) which forces another substance to lose electrons and it itself undergoes reduction by accepting electrons is called oxidizing agent. The oxidizing agent is an electron acceptor e.g. Cl, F, Br, O etc.
Reducing Property:
The tendency of an element to lose electrons is called its reducing property. By virtue of this property, the substance itself undergoes oxidation.
Oxidizing Property:
The tendency of an element to gain electrons is called its oxidizing property. By virtue of this property, the substance itself undergoes reduction.
Factors Affecting the Oxidizing and Reducing Property:
- Ionisation potential
- Electropositivity or electronegativity
- Atomic size
- Metallic and non-metallic character
- Number of valence electrons
Trend in oxidizing and Reducing Property:
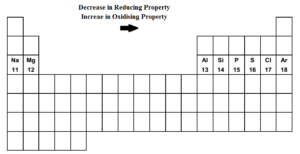
- As we move from left to right i.e. from sodium to chlorine along the third row, the oxidizing property goes on increasing while reducing property goes on decreasing.
- Sodium, magnesium, aluminium are good reducing agents. Silicon, phosphorous and sulphur are weak reducing agents.
- Sodium is the strongest reducing agent. Chlorine is the strongest oxidizing agent. Argon is neither oxidizing agent nor reducing agent.
Scientific Reasons:
As we move from left to right i.e. from Sodium to Chlorine along the third row, the oxidizing property goes on increasing while reducing property goes on decreasing.
Oxidizing and reducing strengths of elements depend upon the atomic size, ionization enthalpy, electropositive and electronegative character, and the number of valence electrons.
Those elements who have bigger atomic size, lower ionization enthalpy, and few valence electrons tend to donate electrons and hence are reducing agents. Hence sodium, magnesium, aluminium are reducing agents.
Those elements who have greater ionization enthalpy, smaller atomic size and more valency electrons tend to accept electrons and hence are the oxidizing agent. Hence Chlorine is an oxidizing agent.
It is observed that, as we move from left to right along the third period atomic size gradually decreases, ionization enthalpy increases and the number of valence electrons increases. Hence electron donating tendency of elements goes on decreasing and that of electron gaining tendency of elements goes on increasing. Hence as we move from left to right i.e. from sodium to chlorine along the third row, the oxidizing property goes on increasing while reducing property goes on decreasing.
Sodium, magnesium and aluminium are good reducing agents.
Oxidizing and reducing strength of elements depends upon the atomic size, ionization enthalpy, electropositive and electronegative character and a number of valence electrons.
Atomic numbers of sodium, magnesium and aluminium are 11, 12 and 13 respectively. They have 1, 2, and 3, valence electrons respectively. Thus removal of 1, 2, 3 electrons from sodium, magnesium, aluminium respectively would give the stable inert gas configuration of neon.
Compared to other third row elements these elements have bigger atomic sizes and lower ionization potentials. Hence they readily lose their valence electrons and are thus strong reducing agents. Reducing strength decrease in the order. Na > Mg > Al.
Sodium is the strongest reducing agent.
Oxidizing and reducing strength of elements depends upon the atomic size, ionization enthalpy, electropositive and electronegative character and the number of valence electrons.
The atomic number of sodium is 11. It consists of a single unpaired electron (3s1). Thus removal of 1 valence electron would give sodium a stable inert gas configuration of neon.
Sodium has the largest atomic size among third low elements and the lowest ionization potential among third-row elements. Hence Sodium readily loses its valence electron and is thus strongest reducing agents.
Na → Na+ + e–
Silicon, phosphorous and sulphur are weak reducing agents.
Oxidizing and reducing strength of elements depends upon the atomic size, ionization enthalpy, electropositive and electronegative character and the number of valence electrons.
Atomic numbers of Silicon, phosphorous and sulphur are 14, 15 and 16 respectively. They have 4, 5, and 6, valence electrons respectively.
They have comparatively smaller atomic size and higher ionization potential. Hence they show less tendency to lose their valence electrons. They are less electropositive. Hence they are weak reducing agents.
They act as weak reducing agents when treated with strong oxidizing agents like fluorine. They also act as a weak oxidizing agent with strong reducing agents like sodium.
Chlorine is the strongest oxidizing agent in third-row elements.
Oxidizing and reducing strength of elements depends upon the atomic size, ionization enthalpy, electropositive and electronegative character and the number of valence electrons.
Chlorine has high electronegativity. The atomic number of chlorine is 17. It consists of seven electrons in the valence shell. It requires only one electron to complete its octet and attain a stable electronic configuration of argon.
Chlorine has the smallest atomic size among third low elements.
Chlorine has very high ionization potential and very high electron affinity.
Hence it has a strong tendency to gain an electron and it is highly electronegative. Hence Chlorine is the strong oxidizing agent.
CI + e– → Cl–
Argon is neither oxidizing agent nor reducing agent.
Oxidizing and reducing strength of elements depends upon the atomic size, ionization enthalpy, electropositive and electronegative character and the number of valence electrons.
Argon is neither electropositive nor electronegative element Atomic number of Argon is 18. It consists of eight electrons in the valence shell. Thus it has completed octet. s orbital and p orbitals are completely filled. Hence it has a stable electronic configuration.
Argon has very high ionization enthalpy. Hence it has a strong tendency not to gain or lose electrons. Hence Argon is neither oxidizing agent nor reducing agent.
Science > Chemistry > Third Row Elements > Oxidizing and Reducing Property of the Third Row Elements