Science > Chemistry > Introduction to Organic Chemistry > Classification of Organic Compounds
In the last artivle we have studied what is organic chemistry? Why it is termed as chemistry of carbon compounds nowaday? In this article we shall study classification of organic compounds. Depending upon the structure organic compounds are classified into two types. viz. open-chain organic compounds or aliphatic organic compounds or Acyclic organic compounds and closed chain organic compounds or cyclic organic compounds
Open Chain Organic Compounds:
Open chain organic compounds are organic compounds that contain an open chain of carbon atoms which may be straight-chain or branched-chain.
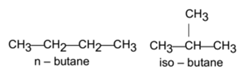
Closed Chain Organic Compounds:
Closed chain organic compounds are organic compounds that contain one or more closed chains or rings of carbon atoms. Closed chain organic compounds are further classified into two types. viz. homocyclic organic compounds or carbocyclic organic compounds. and heterocyclic organic compounds
Homocyclic Carbon Compounds:
Homocyclic organic compounds are organic compounds that contain one or more closed rings of carbon atoms only. Homocyclic organic compounds are further classified into two types. viz. alicyclic organic compounds and aromatic organic compounds
Alicyclic Organic Compounds:
Alicyclic organic compounds are homocyclic organic compounds that have properties similar to that of aliphatic compounds.
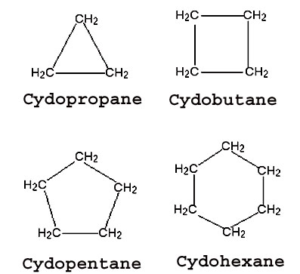
Aromatic Organic Compounds:
Aromatic organic compounds are homocyclic organic compounds that contain at least one benzene ring.
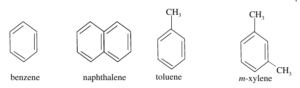
Heterocyclic Organic Compounds:
Heterocyclic organic compounds are organic compounds that contain at least one atom other than a carbon atom in the ring.

Homologous Series:
A series of organic compounds which have a common general formula and in which the two successive members of the series differ by – CH2 – is known as homologous series. The individual member of the homologous series is called homologue.
Alkane Series:
| Sr. No. | Alkane | Molecular Formula | Difference with last alkane | Molecular mass | Difference w.r.t. last alkane |
| 1 | Methane | CH4 | – | 16 | – |
| 2 | Ethane | C2H6 | – CH2 – | 30 | 14 |
| 3 | Propane | C3H8 | – CH2 – | 44 | 14 |
| 4 | Butane | C4H10 | – CH2 – | 58 | 14 |
Alcohol Series:
| Sr. No. | Alcohol | Molecular Formula | Difference with last alcohol | Molecular mass | Difference w.r.t. last alcohol |
| 1 | Mehanol | CH3OH | – | 32 | – |
| 2 | Ethanol | C2H5OH | – CH2 – | 46 | 14 |
| 3 | Propanol | C3H7OH | – CH2 – | 60 | 14 |
| 4 | Butanol | C4H9OH | – CH2 – | 74 | 14 |
Characteristics of Homologous Series:
- Members of the same homologous series are represented by the same general formula. E.g. all alkanes are represented by the same general formula CnH2n + 2.
- They can be prepared by similar methods of preparation.
- They have the same functional group hence have a number of chemical properties in common which are called the general properties.
- They show a regular gradation in physical properties such as melting and boiling points.
- Each member of the homologous series is known as the homologue of the other elements and differs from its next higher or next lower homologue by a common difference –CH2–
- Each member of the homologous series differs from its next higher or next lower homologue in molecular weight by 14.