Science > Chemistry > Introduction to Organic Chemistry > Empirical and Molecular Formulae of Organic Compounds
In this article, we shall study the concept of empirical formula and a molecular formula of a compound.
Empirical Formula:
The empirical formula of a substance represents the simplest relative whole number ratio of the atoms of each element contained in the molecule of the substance.
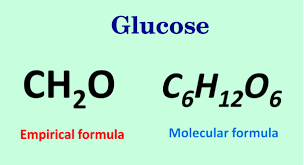
Example: the empirical formula for glucose is CH2O. Thus the molecule of glucose contains atoms of carbon, hydrogen and oxygen in the ratio 1:2:1.
Molecular Formula:
The formula which gives an actual number of atoms of different elements present in the molecule of the compound is called molecular formula.
Example: A molecular formula for glucose is C6H12O6. Thus the molecule of glucose contains 6 atoms of carbon, 12 atoms of hydrogen and 6 atoms oxygen.
The molecular formula is a simple multiple of the empirical formula. The relation between Empirical formula and the molecular formula is given by
Molecular formula = n x Empirical formula
Where n is a whole number and is a ratio of molecular mass to empirical formula mass.
e.g. The empirical formula for glucose is CH2O.
Thus the empirical formula mass of glucose is (12 x 1 + 1 x 2 + 16 x 1 = 30 )
The molecular formula for glucose is C6H12O6
Thus the molecular mass of glucose is (12 x 6 + 1 x 12 + 16 x 6 = 180 )
Now, Molecular formula mass = n x Empirical formula mass
∴ 180 = n x 30
∴ n = 6
Determination of Empirical Formulae/ Molecular Formulae:
Following steps are involved in the determination of empirical formula of organic compound.
- Determination of atomic ratio: The percentage concentration of each element in the compound is divided by its respective atomic weight to get the atomic ratio. It is to be noted that if the sum of the percentage of constituent elements is not 100%, then the rest of the percentage is assumed to be that of oxygen.
- Determination of simplest ratio: The ratio so obtained is divided by the smallest ratio in order to obtain the least ratio or the simplest ratio.
- Determination of whole-number ratio: If the ratios are fractional, reduce them to the smallest possible whole numbers by multiplying throughout by suitable integer. This gives the simplest whole-number ratio.
- Writing the empirical formula: Write down the symbols present side by side with the above numbers as a subscript to the lower right corner of each. This gives the empirical or the simplest formula.
- Find the value of n: Find the value of the ratio of molecular mass to empirical formula mass and denote it as n
- Find molecular formula using relation
Molecular Formula = n x Empirical Formula.
Numerical Problems:
Example – 01:
An organic substance contains 65% carbon, 3.5 % hydrogen and 9.59% nitrogen. Find its empirical formula.
Solution:
The sum of the percentage of carbon, hydrogen and nitrogen is not 100%.
Hence the rest of the part of the compound is oxygen.
Percentage of oxygen = 100 – (65 + 3.5 + 9.59) = 21.91%
| Element | Symbol | Percentage | Atomic weight | Relative No. of atoms | Simple ratio |
| Carbon | C | 65% | 12 | 65/12 = 5.42 | 6.42/0.685=8 |
| Hydrogen | H | 3.5% | 1 | 3.5/1=3.5 | 3.5/0.685=5 |
| Nitrogen | N | 9.59% | 14 | 9.59/14= 0..685 | 0.685/0.685=1 |
| Oxygen | O | 21.91% | 16 | 21.91/15= 1.324 | 1.324/0.685=2 |
The simplest ratio of C to H to N to O is 8:5:1:2
Hence empirical formula = Ans. C8H5NO2.
Example – 02:
An organic compound contains C, H, and O and is found to have 32% carbon, 4% of hydrogen, the remaining being oxygen. What is the molecular formula? If it contains six atoms of oxygen per molecule?
Solution:
The sum of the percentage of carbon and hydrogen s not 100%.
Hence the rest of the part of the compound is oxygen.
Percentage of oxygen = 100 – (32+4) = 64%
| Element | Symbol | Percentage | Atomic weight | Relative No. of atoms | Simple ratio |
| Carbon | C | 32% | 12 | 32/12=2.67 | 2.67/2.67=1 |
| Hydrogen | H | 4% | 1 | 4/1=4 | 4/2.67= 1.5 |
| Oxygen | O | 64% | 16 | 64/16=4 | 4/2.67 = 1.5 |
The simplest ratio is 1 :1.5:1.5 i.e. 2:3:3
Hence emperical formula of compound is C2H3O3.
The compound contains six atoms of oxygen
n = Number of oxygen in molecular formula/Number of oxygen in molecular formula = 6/3 =2
Therefore, molecular formula = 2 x (Empirical formula)
Molecular formula = 2 x (C2H3O3) = C4H6O6
The molecular formula for the compound is C4H6O6
Example – 03:
An organic compound contains 40% carbon, 6.66% of hydrogen. What is the molecular formula of the compound? If its molecular weight is 180.
Solution:
The sum of the percentage of carbon and hydrogen is not 100%.
Hence the rest of the part of the compound is oxygen.
Percentage of oxygen = 100 – (40 + 6.66) = 53.34%
| Element | Symbol | Percentage | Atomic weight | Relative No. of atoms | Simple ratio |
| Carbon | C | 40% | 12 | 40/12=3.33 | 3.33/3.33=1 |
| Hydrogen | H | 6.66% | 1 | 6.66//1=6.66 | 6.66/6.66=2 |
| Oxygen | O | 53.34% | 16 | 53.34/16=3.33 | 3.33/3.33=1 |
Simplest ratio = 1:2:1
Hence empirical formula = CH2O.
Empirical formula weight = 12 x 1 + 1 x 2 + 16 x 1 = 30
n = Molecular mass / empirical formula mass = 180/30 = 6
Therefore, molecular formula = n x (Empirical formula)
Molecular formula= 6 x (CH2O)
Molecular formula = C6H12O6.
Therefore molecular formula of the organic compound is C6H12O6.
Example – 04:
An organic monobasic acid contains 18.6% carbon, 1.55% of hydrogen, 55.04% chlorine, and 24.81% oxygen. What is the molecular formula of the acid, If its molecular weight is 129?
Solution:
The sum of the percentage of carbon, hydrogen, and chlorine is not 100%.
Hence the rest of the part of the compound is oxygen.
Percentage of oxygen = 100 – (18.6 + 1.55 + 55.04) = 24.81%
| Element | Symbol | Percentage | Atomic weight | Relative No. of atoms | Simple ratio |
| Carbon | C | 18.6% | 12 | 18.6/12=1.55 | 1.55/1.55 = 1 |
| Hydrogen | H | 1.55% | 1 | 1.55/1 = 1.55 | 1.55/1.55 = 1 |
| Chlorine | Cl | 55.04% | 35.5 | 55.04/35.5=1.55 | 1.55/1.55 = 1 |
| Oxygen | O | 24.81% | 16 | 24.81/16=1.55 | 1.55/1.55 = 1 |
Simplest ratio = 1:1:1:1
Hence empirical formula = CHClO.
Empirical formula weight = 12 x 1 + 1 x 1 + 35.5 x 1 + 16 x 1 = 64.5
n = Molecular mass / empirical formula mass = 129/64.5 = 2
Therefore, molecular formula = n x (Empirical formula)
Molecular formula = 2 x (CHClO)
Molecular formula = C2H2Cl2O2.
Therefore molecular formula of the organic acid is C2H2Cl2O2.
But the acid is monobasic acid, hence it should contain one – COOH group
i.e. the molecular formula of organic monobasic acid is CHCl2COOH.
Example – 05:
Analysis of Vitamin C shows that it contains 40.92% carbon by mass, 4.58% hydrogen, and 54.50% oxygen. Determine the simplest formula of vitamin C.
Solution:
| Element | Symbol | Percentage | Atomic weight | Relative No. of atoms | Simple ratio |
| Carbon | C | 40.92% | 12 | 40.92/12=3.41 | 3.41/3.41=1 |
| Hydrogen | H | 4.58% | 1 | 4.58/1=4.58 | 4.58/3.41=1.34 |
| Oxygen | O | 54.5% | 16 | 54.5/16=3.41 | 4 3.41/3.41=1 |
The simplest ratio is 1 :1.3:1 i.e. 3:4:3
Hence the simplest formula of vitamin C is C3H4O3.