In the last article, we have seen what is meant by alkanes, their classification, and nomenclature. In this article we shall study preparations of alkanes by different methods.
From Unsaturated Hydrocarbons:
Preparations of Alkanes From Alkenes:
Alkanes are obtained by passing the mixture of alkenes and hydrogen over Raney nickel (Ni), or platinum(Pt) or palladium (Pd) at about 473 K to 573 K, corresponding alkanes are obtained. This is hydrogenation process and is also known as Sabatier and Sanderson reaction.
General Reaction and Examples:
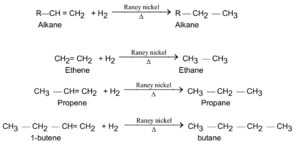
Preparations of Alkanes From Alkynes:
Alkanes are obtained by passing the mixture of alkynes and hydrogen over Raney nickel (Ni), or platinum(Pt) or palladium (Pd) at about 473 K to 573 K, corresponding alkanes are obtained. This is hydrogenation process and is also known as Sabatier and Sanderson reaction.
General Reaction and Examples:
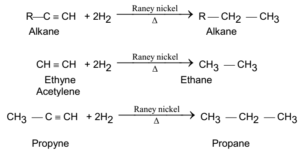
Preparations of Alkanes From Alkyl Halides:
Method – I:
When alkyl halides are treated with a suitable reducing agent like a zinc-copper couple with alcohol, or aluminium – amalgam in water-alcohol or zinc in acetic acid corresponding alkanes are obtained.
General Reaction and Examples:

Method – II (By Wurtz Reaction):
When alkyl halides are treated with active metal like sodium or zinc in presence of dry ether. corresponding alkanes are obtained. In this method, a higher alkane containing a double number of carbon atoms as compared to that in alkyl halide are obtained.
General Reaction and Examples:

From Sodium Salt of Carboxylic Acids (Fatty Acids):
When anhydrous sodium salt of fatty acid is fused with soda lime it gives alkanes containing one carbon less than the carboxylic acid. The process is called decarboxylation.
General Reaction and Examples:

Physical Properties of Alkanes:
- They are colourless, odourless, tasteless compounds.
- The first four alkanes namely, methane, ethane, propane and butane are gaseous at room temperature. Those containing 5 carbons to 17 carbons are liquids with increasing boiling points. Those containing more than 17 carbons are waxy solids.
- They are insoluble in water but soluble in organic solvents like benzene, acetone, ether etc.
- They are lighter than in air and hence float on water.
- They are bad conductors of heat and electricity.
- Boiling points of branched alkanes are lower than that of straight chain alkanes.