In last article, we studied preparations of alkenes. In this article, we shall study reactions of alkanes.
Reactivity of Alkanes (Less Reactivity):
- Alkanes are saturated hydrocarbons. They do not contain any functional group. Hence they are less reactive.
- Under normal conditions, they are not acted upon by concentrated acids, alkalies, oxidising agents etc.
- Branched-chain alkanes are more reactive then straight-chain alkanes.
- Alkanes shows substitution reactions
Reactions of Alkanes:
Halogenation of Alkanes:
Reactivity: F2 > Cl2 > Br2 > I2.
Fluorination of alkanes:
Reaction of fluorine with alkanes highly explosive and highly exothermic. The reaction takes place even in the dark. The reaction is so spontaneous that even the C-C bond is also broken. The reaction of fluorine with alkanes is carried out by diluting fluorine with nitrogen.
Chlorination of alkanes:
Chlorine reacts with alkanes in presence of diffused sunlight in steps by successive replacement of hydrogen by chlorine. A mixture of mono, di, tri and tetrachlorides is obtained.
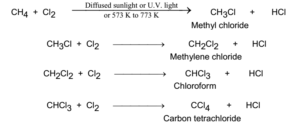
Bromination of alkanes:
A reaction of alkanes with bromine is similar to that of chlorine but the reaction is very slow. Hence it is carried out in presence of AlBr3 as a catalyst.
CH4 + Br2 → CH3Br + HBr
Methane Methyl bromide
Iodination of alkanes:
The reaction of alkanes with iodine is reversible hence direct iodination of alkanes is not possible.
CH4 + I2 ⇌ CH3I + HI
Methane Methyl iodide
If the reaction is carried out in the presence of HIO3, HNO3 or HgO, alkyl halides are obtained.
5CH4 + 2 I2 + HIO3 → 5CH3I + 3H2O
Methane Methyl Iodide
Mechanism of Chlorination of Alkanes:
Chlorination of alkanes is a free radical reaction. The reaction takes in the following steps.
Chain initiation: In presence of sunlight the chlorine molecule is decomposed into chlorine atoms. These atoms are highly reactive as they possess unpaired electron.
In presence of sunlight
Cl–Cl → Cl* + Cl*
Chain propagation: The active chlorine atoms attack methane molecule and remove one hydrogen atom. During this process hydrogen chloride and a free methyl radical is formed.
CH4 + Cl * → CH3* + HCl
Methyl radical formed reacts with another chlorine atom to form methyl chloride and an active chlorine atom. This active chlorine atom again brings about the whole sequence of similar reactions.
CH3* + Cl2 → CH3Cl + Cl *
In this reaction, we can assume one active chlorine atom can bring about an unlimited number of conversions. Hence the reaction is called chain reaction or chain propagation.
Chain termination: Reaction of two species possessing unpaired electrons form neutral molecules. Thus free radicals are consumed and hence these reactions are called chain termination reactions.
Cl * + Cl * → Cl2
CH3* + CH3* → CH3–CH3
CH3* + Cl* → CH3Cl
Nitration of Alkanes:
There is no effect of nitric acid at normal temperature on alkanes. When nitric acid is heated with alkanes to about 423 K to 698 K nitroalkanes are obtained.
General Reaction and Examples:
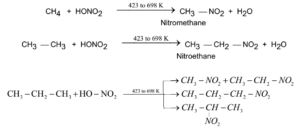
Pyrolysis of Alkanes:
The decomposition of the compound using heat alone (in absence of air) is called pyrolysis In alkanes two types of pyrolysis reaction can take place. Dehydrogenation and cracking.
Cracking of Alkanes:
The decomposition of alkanes by heating to a very high temperature in the absence of air is called the cracking of alkanes. In this process, the carbon-carbon bonds are broken and a mixture of alkane and alkene is obtained.
When propane is heated to about 873 K in the absence of air, ethene and methane is obtained.
CH3–CH2–CH3 → CH2=CH2 + CH4
Propane ethene methane
Dehydrogenation of Alkanes:
The decomposition of alkanes by heating to a very high temperature in the absence of air is called the dehydrogenation of alkanes. In this process, the carbon-hydrogen bonds are broken and a mixture of alkanes and alkenes is obtained.
CH3– CH3 → CH2=CH2 + H2
Ethane Ethene
CH3–CH2–CH3 → CH3 –CH=CH2 + CH2=CH2 + CH4 + H2
Propane Propene Ethene Methane
Aromatization or Reforming Reaction of Alkanes:
Alkanes having more than five carbon atoms get cyclized to benzene and its homologues, on heating under pressure of 10 to 20 atm at about 773 K in presence of oxides of chromium, vanadium or molybdenum supported on alumina.
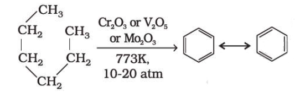
Combustion of Alkanes:
On burning in air alkanes combine with oxygen to form carbon dioxide and water. This reaction is highly exothermic and large amount of heat is liberated during combustion.
CH4 + 2O2 → CO2 + 2 H2O + Heat
C2H6 + 7O2 → 4CO2 + 6 H2O + Heat
Uses of Alkanes:
- Methane is the main constituent of natural gas. Natural gas is used as industrial fuel and for illumination.
- Iso-butane is liquefied petroleum gas (LPG) and is filled in steel cylinders and is used as fuel for cooking and industrial heating.
- Petrol contains a mixture of alkanes containing 6 to 12 carbons, which is used as motor fuel.
- Higher liquid alkanes like kerosene and furnace oil are used as industrial and domestic fuels.
- Diesel is used in a diesel engine.
- Propane is used as refrigerant in petroleum industry.
- A mixture of alkanes containing 20 to 30 carbons is called petroleum jelly or Vaseline and are used in ointments and cosmetics.
- Alkanes containing 17 to 20 carbons are thick liquids which are used as lubricating oils for machines.
- Alkanes are used to manufacture carbon black which is used in paints, inks, boot polish and rubber.
- Methane is used for the synthesis of ammonia
- Methane is used as starting reactant for the manufacture of methanol, methylene chloride, chloroform, carbon tetrachloride, formaldehyde etc.