Science > Chemistry > Surface Chemistry > Adsorption
Adsorption is defined as the phenomenon in which there is the accumulation of one substance on the surface of the other substance. It can also be defined as the change in concentration at the interfacial layer between two phases of the system due to surface forces. OR It can also be defined as the change in concentration at the interfacial layer between two phases of the system due to surface forces.
Examples:
If animal charcoal is shaken with a diluted solution of acetic then it is observed that acetic acid is concentrated on the surface of charcoal. If the ink is shaken with charcoal then the intensity of colour decreases. This is due to the adsorption of ink molecules on the charcoal.
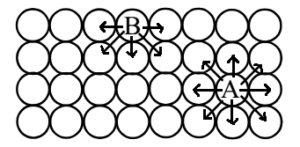
Explanation:
The molecules below the surface of the substance i.e. in the bulk (Molecule A) are equally attracted by other molecules from all sides but molecules on the surface (Molecule B) are subjected to an unbalanced attraction from molecules in the bulk. These unbalanced forces try to drag the molecules inside. So as to satisfy these unbalanced residual forces surface molecules tend to attract and retain particles or molecules of other substances on the surface with which they come in contact.
When a solid surface is exposed to a gas or liquid, the molecules from the gas or liquid accumulate or concentrate at the surface. This process is called as adsorption.
Since the surface molecules are responsible for adsorption, it is a surface phenomenon and its extent depends on the surface area of the absorbent. Adsorption may occur when two heterogeneous phases are in contact with each other.
Terms Used in Surface Chemistry:
Adsorbent:
The substance whose surface adsorbs the gas or solute molecules from solution is called as an adsorbent.
e.g. If animal charcoal is shaken with a dilute solution of acetic then it is observed that acetic acid is concentrated on the surface of charcoal. Thus charcoal is adsorbent.
Adsorbate:
The substance which gets adsorbed on the surface of solid or a liquid is called as adsorbate or adsorbed phase.
e.g. If animal charcoal is shaken with a dilute solution of acetic then it is observed that acetic acid is concentrated on the surface of charcoal. Thus acetic acid is adsorbate.
Heat of Adsorption:
Adsorption is an exothermic process. The heat evolved per mole of adsorbate is called heat of adsorption.
Desorption:
The removal of an adsorbed substance from the surface is known as desorption. It is an endothermic process. i.e. Increase in temperature increases the rate of desorption.
Absorption:
Absorption is a phenomenon in which a substance penetrates through the surface and gets distributed uniformly throughout the body or bulk of another substance. e.g. 1. Water (absorbate) is absorbed by the sponge (absorbent).2. Ammonia gas is absorbed in water.
Difference Between Adsorption and Absorption:
Characteristics of Adsorption:
- It is a surface phenomenon.
- It takes place due to the presence of residual surface forces.
- It is dependent upon temperature and pressure.
- It is affected by the surface area of adsorbent.
- It is in an exothermic process.
- It is a reversible process and a state of dynamic equilibrium is attained.
- Example: Accumulation of acetic acid molecules on the surface by charcoal.
Characteristics of Absorption:
- It is a bulk phenomenon.
- It takes place due to the porous nature of the substance.
- It is independent of temperature and pressure.
- It is not affected by the surface area of the absorbent.
- It is neither exothermic nor endothermic process.
- It is not a reversible process and a state of static equilibrium may be reached.
- Example: Absorption of water by a sponge.
Experiment to Demonstrate Adsorption:
Take about 100 ml of 0.1 N acetic acid solution in a beaker. Add about 5 grams of activated and powdered charcoal to the solution. Stir the solution containing charcoal and keep it standing for 30 minutes. Filter the solution and find the strength of this solution (filtrate) by titration against 0.1 N NaOH.
It is observed that the concentration of an acetic acid solution is decreased as acetic acid is adsorbed by charcoal. This experiment proves that the molecules of acetic acid must be adsorbed by activated charcoal. It is a liquid-solid system in which acetic acid is adsorbate and charcoal is adsorbent.
This is reversible as if the charcoal in above experiment is boiled with water, the adsorbed acetic acid molecules will be desorbed.
Types of Adsorptions:
It is found that the forces operative in adsorption are not the same in all cases. Depending upon the forces which hold the particles or molecules of adsorbate on the surface of adsorbent there are two types of adsorption namely a) Physical adsorption and b) Chemical adsorption or chemisorption.
Physical Adsorption:
The adsorption in which molecules of adsorbate are held on the surface of adsorbent by Van der Waals forces or weak physical forces is called as physical adsorption or Van der Waals adsorption or physisorption.
Examples:
- Accumulation of ammonia gas on the surface of activated charcoal.
- Accumulation of hydrogen gas on the surface by platinized platinum.
- Accumulation of acetic acid on surface by charcoal.
Characteristics of Physical Adsorption:
- In this type, the adsorbed molecules are held on the surface of adsorbent by weak Van der Waals forces.
- As the Van der Waals forces or physical forces are not specific in character, it is said to be general in character.
- Van der Waals forces or physical forces are weak therefore it is reversible and it has a low heat of adsorption.
- Van der Waals forces or physical forces may operate between adsorbed molecules and the other non-adsorbed molecules this makes it multilayer in character.
- The energy of activation is low.
- It takes place at low temperature.
- It adsorption is a fast process.
Chemical Adsorption:
The adsorption in which molecules of adsorbate are held on the surface of adsorbent by chemical forces (chemical bonds) is called as chemical adsorption or chemisorption.
Examples:
- Accumulation of hydrogen gas on nickel.
- Accumulation of oxygen or carbon monoxide on the surface of tungsten.
Characteristics of Chemical Adsorption:
- In this type, the adsorbed molecules are held on the surface of adsorbent by strong chemical bonding forces.
- As the chemical bonds are highly specific in character, it is said to be specific in character.
- Chemical bonds are strong therefore it is irreversible and it has a high heat of adsorption.
- For it to take place, there must be a direct contact between adsorbate and adsorbent molecules, therefore, it results in monolayer formation of adsorbate on the surface of the adsorbent.
- The energy of activation is high.
- It takes place at high temperature.
- It is a slow process.
One reply on “Adsorption”
I really appreciate the effort put in this website.