Science > Chemistry > Surface Chemistry > Application of Adsorption: Catalysis
In this article, we shall discuss very important application of the phenomenon of adsorption, called catalysis. Remaining applications are discussed in the next article.
Catalyst:
A catalyst is defined as a substance which when added to the reacting system increases the rate of the reaction without itself being consumed in the reaction.
e.g. Thermal decomposition of KClO3 is a very slow process. But this decomposition can be carried out even at a lower temperature by heating KClO3 with MnO2 powder. Here MnO2 acts as a catalyst.
Catalysis:
Finely divided metals are used as a catalyst in many gaseous reactions. The catalytic action can be explained as follows. In heterogeneous reactions, catalyst acts as adsorbent and the reactants act as the adsorbate.
In catalytic reactions of gases, the reacting gas molecules are adsorbed on the surface of metal catalysts. Thus concentration of reacting gas molecules increases due to the accumulation of it in a smaller region on the surface of the catalyst. Since according to the law of mass action the rate of chemical reaction is proportional to the concentration of the reactants, the reaction will proceed faster at the surface of the adsorbent.
Similarly adsorption results into weakening of interatomic bonds in the reactant molecules which results in easier rupture of the bonds and into higher activity of reactants
Adsorption is an exothermic phenomenon. Heat evolved during the adsorption helps in exciting adsorbed molecules of reactants. Thus the overall rate of chemical reaction increases.
Examples:
- In the synthesis of ammonia by Haber’s process, finely divided iron is used as a catalyst.
- Finely divided nickel is used as a catalyst in the hydrogenation of oils.
- In preparation of sulphur trioxide from sulphur dioxide, vanadium pentoxide is used as a catalyst.
Characteristics of Catalyst:
- It seems that the catalyst does not take part in the reaction but actually it forms a complex with reactant/ reactants which further regenerates into product/ products and catalyst. Thus
- Reactant / Reactants → Catalyst Complex
- Catalyst Complex → Product / Products + Catalyst
- Thus catalyst is recovered at the end of the reaction.
- In reversible reactions, the catalyst increases the rate of both the forward reaction and the backward reaction. Thus equilibrium is not influenced by the presence of a catalyst.
- An extremely small quantity of catalyst causes a considerable increase in the rate of reaction.
- The activation energy of catalysed reaction is always lower than that of the same reaction when it is uncatalysed.
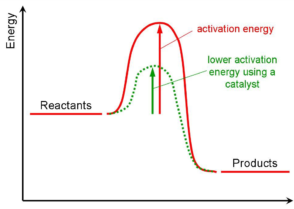
- A catalyst does not affect the energies of reactants and products. Hence the heat of reaction is the same for catalysed and uncatalysed reaction.
- The substances which inhibit the catalytic activity are called catalytic poisons. In case of conversion of SO2 into SO3 catalytic activity of platinum is totally destroyed by the presence of small traces of arsenic due to the formation of platinum arsenide. Thus, in this case, arsenic is catalytic poison.
- A catalyst increases the rate of reaction but they don’t initiate the reaction.
Inhibition or Retardation of a Reaction:
A substance that decreases the rate of a chemical reaction is called an inhibitor. The phenomenon in which the rate of a chemical reaction is reduced is called inhibition or retardation. For Example:
- Chloroform reacts with atmospheric air to form poisonous carbonyl chloride. Thus the use of chloroform as an anaesthetic is dangerous to life. To avoid this reaction or to reduce the rate of the reaction 2% ethanol is added to chloroform. Thus ethanol acts as an inhibitor.
- Hydrogen peroxide decomposes itself and thereby reduces in strength. This decomposition can be retarded by adding dilute acid or glycerol to it. Thus the dilute acid or glycerol acts as an inhibitor.
Classification of Catalysis:
On the Basis of the phases of catalyst and reaction mixture:
Homogeneous catalysis:
A homogeneous catalysis is one in which the catalyst and the reactants exist in the same phase. A homogeneous catalyst dissolves in the gas phase or solution and acts uniformly throughout.
Examples:
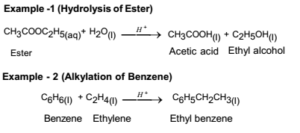
Other Examples:

Characteristics of Homogeneous Catalysis:
- The catalyst and the reactants form a single phase.
- The catalyst dissolves into the gas phase or solution.
- The reaction occurs in the gas phase or liquid phase.
- The catalyst is often involved in the chemical reaction.
- The catalyst cannot be easily separated from the products of the reaction.
- The rate of reaction does not depend on the surface area of the catalyst.
- These reactions are generally faster than heterogeneous catalysis.
Heterogeneous catalysis:
A catalyst which exists in a different phase from the reactants is known as a heterogeneous catalyst and the catalysis known as heterogeneous catalysis. Generally, the Heterogenous catalysts are in a solid state, while the reactants are in the liquid or gaseous state.
Examples:


Characteristics of Heterogeneous Catalysis:
- The catalyst and the reactants form different phases.
- The catalyst does not dissolve into reacting mixture.
- The reaction does not occur in the gas phase or liquid phase but takes place on the surface of the catalyst.
- The catalyst is not involved in the chemical reaction. It absorbs the reactants on its surface.
- The catalyst can be easily separated from the products of the reaction.
- The rate of reaction depends on the surface area of the catalyst.
- These reactions are generally slower than homogeneous catalysis.
Steps Involved in Heterogeneous Catalysis:
- The reactant molecules diffuse to the surface of the solid catalyst.
- Reactants molecules are adsorbed on the surface of the catalyst by chemical bonding between surface molecules and the reactant molecules.
- The reactants are converted into products on the surface of the catalyst.
- The product molecules leave the catalyst surface. i.e. they are desorbed.
- The product molecules then diffuse into the gaseous phase.
Catalytic Activity:
The activity of catalyst depends on the strength of chemical adsorption. When a solid catalyst is highly covered by the adsorbate, the chemisorption is said to be strong and the catalyst is then active. If this chemisorption is too strong the adsorbate molecules become motionless on the surface, thus the activity of reacting substance decreases. Thus very strong chemisorption weakens the activity of the catalyst. Thus the adsorbate should get adsorbed strongly but not so strong that their activity reduces.
The metals which lie close to the middle of d block of the periodic table are the most active catalyst.
Catalytic Selectivity:
Different catalysts with same reacting mixture give different product. Selectivity of catalyst is its tendency to catalyse the reaction to form particular products.

Enzyme Catalysis:
Enzymes are the biological homogeneous catalyst. They are large protein molecules. They have large complex structure. Enzymes are very efficient catalysts under very mild conditions in comparison with another type of catalysts. This is because the reduction in activation energy is much greater than that of other types of catalysts.
The enzymes are highly specific in their action. They catalyse only a single reaction of a single compound.For example, the enzyme amylase catalyses the conversion of starch into glucose but don’t have any effect on cellulose.
