Science > Chemistry > Surface Chemistry > Applications of Adsorption
In this article, we shall study some applications of adsorption.
Adsorption Indicators:
The phenomenon of adsorption is used to detect the endpoints of precipitation titrations. In such titrations, dyestuffs like eosin, fluorescein, alizarin red etc, are used as adsorption indicators. At the endpoint of the titration anions of indicator adsorb on precipitate and colour change of the precipitate takes place.
When a known volume of KBr (in a conical flask) is titrated against AgNO3 (in burette) using eosin as adsorption indicator, AgBr a white precipitate is formed.
AgNO3 + KBr → Ag+Br– + KNO3
There is no colour change of the precipitate as long as Br– ions are present in the solution. Before endpoint AgBr precipitate is in contact with unreacted KBr and therefore it will adsorb Br– ions and negatively charged (AgBr)Br– are formed. The negatively charged precipitate will repel anions of eosin which are pink in colour. Colour of precipitate remains unchanged.
When all Br– ions are consumed i.e. entire KBr is converted into AgBr, at this stage, an excess drop of AgNO3 results into adsorption of Ag+ ions and (AgBr) Ag+ are formed. These particles immediately adsorb the coloured anions of the indicator eosin and colour of the precipitate changes to pink. This is the end of the titration.
Adsorption Chromatography:
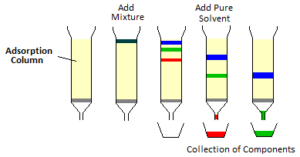
Different constituents of a mixture can be separated by adsorption chromatography technique. The chromatographic technique is based on the fact that, the different constituents of the mixture will adsorb to a different extent on the adsorbent due to their varying adsorption affinity. This is called as preferential or selective or differential adsorption.
In chromatography technique, the mixture to be separated is first dissolved in a suitable solvent like acetone, benzene, ether etc. The solution is then allowed to pass through a glass tube containing adsorbent like silica gel, alumina, cellulose, resins etc. called adsorbing column. The different constituents are adsorbed preferentially at different layers (bands). The most readily adsorbed constituents form the upper most band while the least readily adsorbed constituents form the lowermost band. In this way, all constituents are separated making different bands. A pure solvent is then poured, different bands are dissolved and collected separately in the form of a solution. This is called elution.
For e.g. in column chromatography, a glass tube is filled with a slurry of alumina and water. a solution of a mixture containing ions of Fe+++, Cu++, Co++ etc. is added to the top of the column and there is a phenomenon of differential adsorption.
These adsorbed ions are then washed (Elution) with HCl solution (Eluent). Now Co++ de-adsorb first and are collected in one receiver Then Cu++ are de-adsorbed and finally Fe+++ are de-adsorbed and collected in separate receivers. Thus we can conclude that the ions which are readily adsorbed are least readily or reluctantly de-adsorbed.
Chromatography is extensively used in the separation of metallic salts from their mixture using ion exchange resins.
Water Purification:
Using charcoal:
Naturally, occurring water is impure. It is contaminated with soluble and insoluble impurities. When impure muddy water is passed through a bed of activated charcoal. Many impurities like vegetable and colouring matter get adsorbed on charcoal and water is purified.
Charcoal has a double action. Its porous nature acts as a filter for removing insoluble impurities in impure water. It acts as adsorbent and adsorbs dissolved impurities from impure water.
Using alum:
Impurities in water can be removed by adding alum. Alum is a good coagulating agent, so the colloidal impurities precipitate easily. Alum forms a gelatinous precipitate of positively charged colloidal AI(OH)3 which is good adsorbent. It absorbs impurities and colouring matter particularly negatively charged particles and by mutual coagulation. Both settle down and supernatant water becomes clear.
Using ion-exchange resins :
De-ionisation of water by ion-exchange resins is also considered to be adsorption phenomenon. The cation exchange resins adsorb cations like Ca++, Mg++ and exchange H+ ions. The anion exchange resin adsorbs anions like Cl–, SO4— and exchange OH– ions. Thus all the ions except H+ and OH– are removed from the water and the pure water called de-ionised water is obtained.