Science > Chemistry > Colloids > Colloidal Solutions
Depending upon the size of particles in solution, solutions are classified as true solutions, colloidal solutions, and suspensions. In this article, we shall study colloids i.e. colloidal solutions.
Historical Background:
The foundation of colloidal chemistry was laid by English chemist Thomas Graham in 1861. Other prominent chemists contributed to this field are Tyndall, Hardy, Zsigmondy, N. R. Dhar, S.S. Bhatnagar. Thomas Graham classified the soluble substances into two categories. namely, a) crystalloids and b) colloids
Crystalloids:
Many crystalline substances like common salt, sugar, urea when dissolved in water can pass through parchment membrane are termed as crystalloids and their solution with water is called as a true solution. In true solution particle size of solute is very small. The diameter of the particles is about 1 × 10-9 m. They cannot be seen by any instrument.
Colloids:
The substances like gum, glue which when dissolved in water do not pass through a parchment membrane. A solution of colloidal substance with water is known as a colloidal solution. In colloidal solution particle size varies from 5 × 10-9 m to 2 × 10-7 m. in diameter. Colloidal particles can be seen clearly under a high-resolution microscope or ultramicroscope. Any substance can be converted into a colloidal state by suitable means.
Types of Solutions on the Basis of Size of Particles:
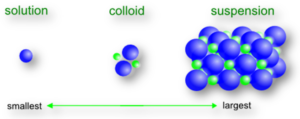
True Solution:
Crystalline substances like sugar, sodium chloride are called crystalloids and are soluble in water. The aqueous solution of crystalloids is called a true solution.
Characteristics of True Solutions:
- It is a homogeneous solution as the particles are not seen even under ultramicroscope.
- The solution is clear and transparent.
- The solution is a single phase, consisting of solute (sugar, sodium chloride) and solvent (water)
- Size of solute particles is 1 × 10-9 m. Therefore they can pass through both the parchment membrane as well as through filter paper.
- The particles can be neutral molecules as in the case of sugar or contain cations (Na+) and anions (Cl–) as in case of NaCl.
- The true solution does not exhibit the Tyndall effect. When a powerful beam of light is passed through a true solution kept in a dark, the path of the beam of light through the true solution is invisible.
- A true solution does not exhibit Brownian movement. The zig-zag motion of particles like pollen grains in a random direction in straight lines is called Brownian movement.
- Particles of solute never settle down under gravity.
- They are not visible by any optical means.
- They can diffuse rapidly.
Colloidal Solution or Colloidal Dispersion:
The word colloids is derived from the Greek word Kolla (glue) and oid (like). Thus colloid means glue-like. When a thin paste of amorphous substances like glue, gum or starch etc. is added to boiling water, taken in a beaker, with continuous stirring a solution obtained is called as a colloidal solution or colloidal dispersion.
Characteristics of Colloidal Solutions:
- It is a heterogeneous solution consisting of two immiscible phases. They are visible under ultramicroscope.
- The solution is turbid.
- There are two phases present in the solution. One phase is present in the form of small particles, dispersed in the medium is called as the dispersed phase or discontinuous phase. The other phase in which particles are dispersed is called as dispersion medium or continuous phase.
- The particle size of the dispersed phase (diameter) is in the range of 5 × 10-9 m to 2 × 10-7 m. Hence they can pass through filter paper but not through parchment membrane.
- All colloidal particles in solution carry the same charge either positive or negative. Due to same nature of charge on all colloidal particles in the solution, the particles repel each other and thus impart stability to the solution.
- Colloidal solutions exhibit the Tyndall effect
- Colloidal solutions exhibit Brownian movement
- Particles do not settle under gravity.
- They diffuse slowly.
Suspension:
When sand is stirred into water, the solution obtained is called a suspension.
Characteristics of Suspension:
- It is a heterogeneous solution consisting of two immiscible phases. They are visible to the naked eye.
- The solution is turbid.
- There are two phases present in the solution. One phase is present in the form of small particles. Dispersed in the medium is called as the dispersed phase or discontinuous phase. The other phase in which particles are dispersed is called as dispersion medium or continuous phase.
- The dispersed particles are aggregates of millions of molecules. The diameter of the particles are aggregates of millions of molecules. The diameter of particles is greater than 10 -7 m. Hence they can not pass through filter paper and not through a parchment membrane.
- Particles do not carry any charge.
- Suspensions do not exhibit Tyndall effect
- Suspensions do not exhibit Brownian movement
- Particles settle under gravity.
- They do not diffuse.
Note:
The colloidal state is intermediate between crystalloids and suspensions. The colloidal solution is a solution of colloidal substance. The colloidal solution is consists of two phases.
The Terminology of Colloidal Solutions:
Colloidal State:
The colloidal state is a heterogeneous dispersion of two immiscible phases which possess certain distinguishing characteristics.
Colloidal Solution:
The colloidal solution is a heterogeneous system consisting of two phases.
Dispersed Phase:
The colloidal substance which is dispersed in a solvent is called as dispersed phase or inner phase or internal phase or discontinuous phase.
e.g. In smoke, carbon particles are dispersed phase.
Dispersion Medium :
The medium in which the colloidal substance is dispersed is known as dispersion medium or outer phase or external phase or continuous phase.
e.g. In starch solution, water is a dispersion medium. another e.g. for a copper sol, copper particles constitute dispersed phase and water the dispersion medium.
Applications of Colloids:
Foods:
- Majority of our food material are colloidal in nature.
- Emulsion -Milk, Cod Liver oil, Buttermilk
- Gels – Jelly, Curd, Butter, Cheese,
- Juicy fruits like mango, apple,
- Badami halva.
- Solid foam – Biscuits, Cake, Bread, Dryfruits
- Foam – Whipped cream
Medicines:
- A number of medicines are colloidal. Colloidal medicines are easily assimilated by body tissues.
- Argyrol and protargol are protected colloidal solutions of silver is used against granulation
- Colloidal gold, calcium injections are used to raise the vitality of the human physiological system against diseases like T.B. and rickets.
- Colloidal sulphur is used as germs killer in plants
- Colloidal antimony is used in curing kala-azar
- Milk of magnesia is used in the treatment of stomach acidity.
Industrial Goods:
- Many industrial goods in our daily life are colloids. e.g. soaps, toothpaste, gum, shoe polish, enamels, resins, leather, paints, varnishes, cosmetics, etc.