Science > Chemistry > Physical Chemistry > Ionic Equilibria >Buffer Solutions
In this article, we shall study the concept of buffer solution, its characteristics, its types, and preparations.
Buffer Solution:
A solution, which resists the change in its pH value, even on the addition of a small amount of strong acid or base is called a buffer solution or buffer.
Example: Mixture of acetic acid (CH3COOH) and Sodium acetate CH3COONa in water.
Characteristics of Buffer:
- It has a definite pH value.
- Its pH value doesn’t change on keeping for a long time
- Its pH value doesn’t change on dilution.
- Its pH value doesn’t change even with the addition of a small amount of a strong acid or a base.
Buffer Action:
The property of the solution to resist the changes in its pH value on the addition of small amounts of strong acid or base is known as buffer action.
Types of Buffer:
Acidic Buffer:
A mixture of a weak acid and its salt of a strong base in water is called an acidic buffer. The pH value of acidic buffer is less than 7.
Preparation: Acidic buffer is prepared by mixing weak acid and its salt with a strong base in a water medium.
Examples: CH3COOH + CH3COONa (the mixture of acetic acid and sodium acetate in water) and HCOOH + HCOONa (the mixture of formic acid and sodium formate in water)
Basic Buffer:
A mixture of a weak base and its salt of strong acid in a water medium is called a basic buffer. Its pH value is greater than 7.
Preparation: It is prepared by mixing a weak base and its salt of strong acid in a water medium.
Examples: NH4OH + NH4CI (the mixture of ammonium hydroxide and ammonium chloride in water) and NH4OH + NH4NO3 (the mixture of ammonium hydroxide and ammonium nitrate in water in water)
A Single Salt Solution:
When a single salt of a weak acid and a weak base is dissolved in water a buffer solution is obtained. Its pH value depends on the relative strength of the weak acid and weak base.
Preparation: It is prepared by mixing single salt of a weak acid and a weak base in water.
Examples: CH3COONH4 (ammonium acetate)
Notes:
- Strong Acid Buffers: Strong acids such as nitric acid, hydrochloric acid or sulphuric acid can act as a buffer with low pH. As these acids are almost completely ionized, the concentration of hydrogen ions is high. The addition of a small amount of acid or base to these acids will have a negligible effect on the pH of the solution.
- Strong Base Buffers: A strong base such as sodium hydroxide, potassium hydroxide can act as a buffer with high pH. As these bases are almost completely ionized, the concentration of hydroxyl ions is high. The addition of a small amount of acid or base to these bases will have a negligible effect on the pH of the solution.
Mechanisms of Buffer Action:
Mechanism of Buffer Action of Acidic Buffer:
The property of the solution to resist the changes in its pH value on the addition of small amounts of strong acid or base is known as buffer action.
Consider an acidic buffer, a mixture of acetic acid (CH3COOH) and sodium acetate (CH3COONa). In an aqueous medium, CH3COOH and CH3COONa dissociates as,
CH3COOH(aq) ⇌ CH3COO–(aq) + H+(aq) (Slight ionisation)
CH3COONa(aq) → CH3COO– aq) + Na+(aq) (Complete ionisation)
If a strong acid like HCI is added to the buffer solution, the additional H+ ions combine with the acetate ions in the solution to produce undissociated CH3COOH.
H+(aq) + CH3COO–(aq) → CH3COOH(aq)
Since additional H+ ions of acid are consumed (neutralized), the pH of the solution remains unchanged. This resistance to change in pH on the addition of a strong base is called as reserve basicity and is due to CH3COO– ions.
If strong base like NaOH is added to the buffer solution, additional OH– ions combine with CH3COOH as
NaOH(aq) + CH3COOH(aq) → CH3COONa(aq) + H2O
OH–(aq) + CH3COOH(aq) → CH3COO–(aq) + H2O
Since additional OH– ions of the base are consumed or neutralized, the pH of the solution remains unchanged. This resistance to change in pH on the addition of base is called reserve acidity and is due to CH3COOH.
Mechanism of Buffer Action of Basic Buffer:
The property of the solution to resist the changes in its pH value on the addition of small amounts of strong acid or base is known as buffer action.
Consider a basic buffer, the mixture of Ammonium hydroxide (NH4OH) and Ammonium chloride (NH4Cl) In an aqueous medium NH4OH and NH4Cl dissociates as
NH4OH(aq) ⇌ NH4+(aq) + OH–(aq) (Slight ionisation)
NH4Cl(aq) → NH4+(aq) + Cl–(aq) (Complete ionisation)
If a strong acid like HCI is added to the buffer solution, additional H+ ions of acid combine with NH4OH, to produce ammonium ions and water.
HCl(aq) + NH4OH(aq) → NH4Cl(aq) + H2O
H+(aq) + NH4OH(aq) → NH4+(aq) + H2O
Since additional H+ ions of acid are consumed (neutralized), the pH of the solution remains unchanged. This resistance to the change in pH upon the addition of strong acid is called reserve basicity and is due to NH4OH molecules.
If a strong base like NaOH is added to the buffer solution, additional OH– ions of the base combine with NH4+ ions to produce undissociated NH4OH molecules.
OH– (aq) + NH4+ (aq) → NH4OH(aq)
Since additional OH– ions of the base are consumed (neutralized) pH of the solution remains unchanged. This resistance to change in pH on addition base is called as reserve acidity and is due to NH4+ ions in a solution.
Mechanism of Buffer Action of Single Salt Solution:
The property of the solution to resist the changes in its pH value on the addition of small amounts of strong acid or base is known as buffer action.
Consider a single salt buffer solution of ammonium acetate (CH3COONH4). In an aqueous medium CH3COONH4 dissociates as,
CH3COONH4(aq) ⇌ CH3COO–(aq)+ NH4+ (aq)
If a strong acid like HCI is added to the buffer solution, additional H+ ions of acid combine with CH3COO–, to produce practically undissociated CH3COOH
H+(aq) + CH3COO–(aq) → CH3COOH(aq)
Since additional H+ ions of acid are consumed (neutralized), the pH of the solution remains unchanged. This resistance to the change in pH upon the addition of strong acid is called reserve basicity and is due to CH3COO– ions.
If a strong base like NaOH is added to the buffer solution, additional OH– ions of base combine with NH4+ ions to produce practically undissociated NH4OH molecules.
OH– (aq) + NH4+(aq) → NH4OH(aq)
Since additional OH – ions of the base are consumed (neutralized) pH of the solution remains unchanged. This resistance to change in pH upon addition base is called as reserve acidity and is due to NH4+ ions in a solution.
Reserve basicity:
The resistance of a buffer solution to change in pH upon addition of a strong acid is called ‘reserve basicity’
Reserve acidity:
The resistance of a buffer solution to change in pH upon addition of a strong base is called ‘reserve basicity’
pH of a Buffer Solution:
pH of a buffer solution is calculated by applying the Henderson-Hasselbalch equation. Let us consider an acidic buffer consisting of weak acid HA and its salt NaA
Consider dissociation of the acid
HA + H2O ⇌ H3O++ A– (Slight ionisation)
NaA → Na+(aq) + A– (aq) (Complete ionisation)
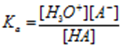
Now the salt NaA is completely dissociated. Hence [A–] = [NaA] = [Salt]. and as HA is almost non dissociated [HA] = [Acid]

Similarly for the basic buffer
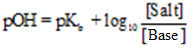
Application of Buffer Solution:
- Buffers are used in the laboratory. In inorganic qualitative and quantitative analysis.
- It is often necessary to adjust the pH of solutions by the calorimetric method.
- They are used in various industrial process viz electrodeposition of metals, tanning of leather, brewing of alcohols, manufacture of paper, etc.
- They are used in the pathological analysis.
- Buffers are also used as stabilizers and preservatives e.g. sodium citrate is used to stabilize penicillin preparations, while sodium benzoate is used as a buffer to preserve jams and jellies. Sulphate preparations are preserved by acetate or acetate buffers,
- Buffers are used to maintain the pH of the soil for a particular crop or horticulture.
- The chemical changes occurring in life processes take place at a definite pH. e.g. the pH of the blood of normal human beings is 7.4. The electrolyte present in the blood act as a buffer solution to maintain the desired value of the pH of the blood.
5 replies on “Buffer Solutions”
My old knowledge of inorganic chemistry is refreshed.
Good explanation
This is informative
thanks for the concised explanations
You not perfect because perfection is of God keep on trying you are good