Science > Chemistry > Solid State > Classification of Crystalline Solids
In this article, we have to study classification of crystalline solids and characteristics of each type. Broadly crystalline solids are classified into 4 types. a) molecular solids, b) ionic solids, c) metallic solids, and d) covalent solids
Molecular Solids:
Molecular solids are crystalline solids in which lattice points are molecules which are held together by means of weak physical forces (van der Waal’s forces). In molecular solids, individual molecules are repeating units. Examples: Phosphorous, sulphur, chlorine and argon are molecular solids because lattice points are molecules.
Characteristics of Molecular Solids:
- In molecular solids the units occupying lattice points are molecules
- The molecules are attached to each other by weak Vander wall’s forces of attraction.
- In these solids, the atoms are joined together within the molecule by strong covalent bonds.
- In these solids, vacant valency orbitals are not available. All the valence orbitals are used for intra-molecular strong covalent bonding.
They have greater ionization enthalpy. - There is strong covalent bonding within the molecules, keeping the atoms together. Due to intermolecular covalent bonds electrons are localized. Hence molecular solids are bad conductors of heat and electricity.
Classification of Molecular Solids:
Molecules of the same compound are the constituent particles of molecular solids. Depending upon the type of molecules involved in crystallization and the nature of intermolecular forces of attraction between the neighbouring molecules, the molecular solids are further subdivided into the following categories:
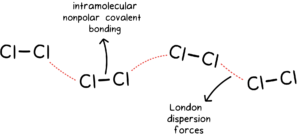
Non-polar Molecular Solids:
- They comprise either atoms like those as noble gases, for example, argon and helium or the molecules formed by non-polar covalent bonds, for example, CH4, H2, Cl2 and I2.
- In these solids, the atoms or molecules are held by weak dispersion forces or van der Wall forces or London forces.
- These solids are soft and non-conductors of electricity.
- They have low melting and boiling points and are usually in liquid or gaseous state at room temperature and pressure.
Polar Molecular Solids:
- The molecules of substances like HCl, SO2, etc. are formed by polar covalent bonds.
- The molecules in such solids are held together by relatively stronger dipole-dipole interactions.
- These solids are soft and non-conductors of electricity.
- Their melting points are higher than those of nonpolar molecular solids yet most of these are gases or liquids under room temperature and pressure.
- They possess a permanent dipole moment. The molecules in these solids are bonded together by stronger dipole-dipole interaction.
- Solid SO2 and solid NH3 are some examples of such solids.
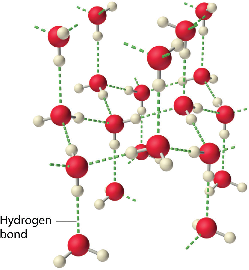
Hydrogen-Bonded Molecular Solids:
- The molecules of such solids contain polar covalent bonds between H and F, O or N atoms.
- Strong hydrogen bonding binds molecules of such solids like H2O (ice), ammonia.
- They are non-conductors of electricity.
- Generally, they are volatile liquids or soft solids under room temperature and pressure.
- Liquids solidify only on cooling.
Ionic Solids:
Ionic solids are crystalline solids in which the units occupying lattice points are positively and negatively charged ions. In such solids the rep[eating units are the positively and negatively charged ions.
Examples: Salts like NaCl, BaSO4, potassium bromide, copper nitrate, copper sulphate are ionic solids.

Characteristics of Ionic Solids:
- Ions are the constituent particles of ionic solids.
- Each ion is surrounded by a number of oppositely charged ions. This number is called coordination number. The coordination number for positive and negative ion may be the same or different.The general coordination numbers for ionic solids are 8, 6 and 4.
- The ionic salts are formed by molecules containing positively charged smaller in size cations and negatively charged relatively bigger anions.
- The charges on cations and anions balance each other hence the solid is electrically neutral.
- Such solids are formed by the three-dimensional arrangements of cations and anions bound by strong coulombic (electrostatic) forces.
- These solids are hard and brittle in nature.
- They have high melting and boiling points.
- They have high density due to close packing.
- Since in solid-state the ions are not free to move about, (due to strong electrostatic force) they are electrical insulators in the solid-state. However, in the molten state or when dissolved in water, the ions become free to move about and they conduct electricity.
Metallic Solids:
Metallic solids are crystalline solids in which the units occupying lattice points are positive ions surrounded by a pool of electrons. (Concept of metallic bond). Examples: The metals Na, Mg, Al are metallic solids.

Characteristics of Metallic Solids:
- In metallic solids, the units occupying lattice points are metal cations, surrounded by many mobile electrons.
- They are good thermal and electrical conductor, malleable and ductile and are lustrous. This is also due to the presence of free electrons in them.
- The atoms in a metal are held together by means of a special type of bond called the metallic bond.
- Their ionization enthalpies are low.
- Their valence electrons are loosely held together and more vacant valency orbitals are available.
The Concept of Metallic Bond:
Metals are an orderly collection of positive ions (called kernels) surrounded by and held together by a sea of free electrons(delocalized). These electrons are mobile and are evenly spread out throughout the crystal. The malleability and ductility of metals are due to the adjustment of the sea of electrons to the new arrangement of kernels in the solid.

Each metal atom contributes one or more electrons towards this sea of mobile electrons. These free and mobile electrons are responsible for the high electrical and thermal conductivity of metals. When an electric field is applied, these electrons flow through the network of positive ions. Similarly, when the heat is supplied to one portion of the metal, the thermal energy is uniformly spread throughout by free electrons.
Covalent Solids:
Covalent solids are crystalline solids in which unit lattice points are atoms. Examples: Diamond, Silicon, silicon carbide (SiC), aluminium nitride (AlN), etc.
Characteristics of Covalent Solids:
- In covalent solids, the units occupying lattice points are atoms attached to each other by covalent bonding. They are also called giant molecules.
- Covalent solids are three-dimensional network solids.
- The crystal of covalent solids consists of a network of chemically bonded atoms.
- Covalent bonds are strong and directional in nature, therefore atoms are held very strongly at their positions. Such solids are very hard and brittle.
- They have extremely high melting points and may even decompose before melting.
- They are insulators and do not conduct electricity.
Graphite:

Graphite is soft and a conductor of electricity. Its exceptional properties are due to its typical structure. Carbon atoms are arranged in different layers and each atom is covalently bonded to three of its neighbouring atoms in the same layer. The fourth valence electron of each atom is present between the different layers and is free to move about. These free electrons make graphite a good conductor of electricity. Different layers are bonded to each other by van der Walls’forces. The distance between the two layers is 3.35 Å. Hence the layers can slide one over the other. This makes graphite a soft solid and a good solid lubricant.
Diamond:

Carbon has an electronic configuration of 2,4. In diamond, each carbon shares electrons with four other carbon atoms forming four single bonds. This is a giant covalent structure. It continues on and on in three dimensions. It is not a molecule, because the number of atoms joined up in a real diamond is completely variable depending on the size of the crystal.
Diamond has a very high melting point (almost 4000°C). Very strong carbon-carbon covalent bonds have to be broken throughout the structure before melting occurs. Diamond is very hard. This is again due to the need to break very strong covalent bonds operating in three dimensions. Diamond doesn’t conduct electricity. All the electrons are held tightly between the atoms and aren’t free to move.
Diamond is insoluble in water and organic solvents. There are no possible attractions which could occur between solvent molecules and carbon atoms which could outweigh the attractions between the covalently bound carbon atoms.
Buckminster fullerene or fullerene:

In 1985 a new allotrope of carbon (C60) was discovered in carbon soot. Sixty carbon atoms form the shape of a ball like a football with a carbon atom at each corner of the 20 hexagons and 12 pentagons. Each carbon atom (shown as a sphere) has three bonds.
The size of the molecule is almost exactly 1 nm in diameter. These are not called giant molecules because there are only sixty atoms. A large number of these molecules can fit together to form a transparent yellow solid called fullerite.
This form of carbon was named after the American architect Buckminster Fuller, who was famous for designing a large dome which looked similar (sort of) to the molecular structure of C60. Many other balls of carbon called fullerenes have since been made, including C70, C76, and C84. These molecules have become known as “buckyballs”.
Fullerenes are used as catalysts and lubricants. They are also used in nanotubes for strengthening materials (for example sports equipment) and are sometimes used as a way of delivering drugs.