Science > Chemistry > Solid State > Defects in Crystal Structure
In this article, we shall study defects in the crystal structure, sources of defects and their types.
Source of Defects in Crystal Structure:
At absolute zero, crystals tend to have a tendency to have a perfectly ordered arrangement. This arrangement at absolute zero represents the lowest energy state of the crystal. As the temperature increases, there is a change in the orderly arrangement of constituents in the crystal. The defects in crystal structure are basically irregularities in the arrangement of constituent particles. Any deviation from the perfectly ordered arrangement constitutes a defect or imperfection. Such defects which are due to temperature change are referred as thermodynamic defects. The imperfection also may be due to impurities present in solid.
The defects in the crystal due to the irregularities in the arrangement of atoms or ions are called atomic imperfections. They are due to missing or misplaced ions. Such defects are referred as point defects. Point defects are the irregularities or deviations from ideal arrangement around a point or an atom in a crystalline substance
If the deviation from periodicity extends over microscopic regions of crystal then they are called lattice imperfections. If lattice imperfections extend along the line they are called line defects. The line defects are the irregularities or deviations from an ideal arrangement in entire rows of lattice points. They are also called dislocations. If the defects extend along a plane they are called plane defects. These imperfections in crystal lead to modification of some properties of the solid or may give rise to new properties.
Usually a solid consists of an aggregate of a large number of small crystals. These small crystals have defects in them. This happens when the crystallization process occurs at the fast or moderate rate. Single crystals are formed when the process of crystallization occurs at an extremely slow rate. Even these crystals are not free of defects.
Point Defects in Crystal Structure:
Point defects are the irregularities or deviations from ideal arrangement around a point or an atom in a crystalline substance. Point defects can be classified into three types:
Stoichiometric Defects in Crystal Structure:
The compound in which the number of positive and negative ions are exactly in the ratios indicated by their chemical formulae are called stoichiometric compounds. The point defects that do not disturb the stoichiometry of the solid are called stoichiometric defects. They are intrinsic or thermodynamic defects. The electrical conductivity of crystal increases due to this defect. These defects are of two types, vacancy defects and interstitial defects.
Vacancy Defects or Schottky Defects:
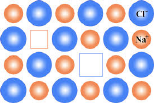
When some of the lattice sites are vacant, the crystal is said to have vacancy defect. The defect produced due to vacancies caused by an absence of anions and cations in the crystal lattice of ionic solid is called a Schottky defect. Thus in such defect, one positive ion and one negative ion are missing from their respective positions leaving behind a pair of holes. This defect can also develop when a substance is heated.
Due to this defect, the observed density of crystal is found to be lower than the expected density. This defect is observed in ionic compounds with high coordination number, high radius and with cations and anions have almost equal size. like NaCl, KCl, CsCl, AgBr, KBr, etc.
Vacancy defects increase with the increase in temperature. It can be observed w.r.t. NaCl crystal as given in the table
| Temperature | Defects |
| Room Temp. (298 K) | 1 in 1015 lattice sites |
| 775 K | 1 in 106 lattice sites |
| 1075 K | 1 in 104 lattice sites |
Interstitial Defects or Frenkel Defects:
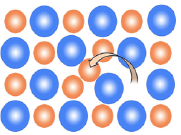
When cation and anion from ionic solid leave its regular site and moves to occupy a place between the lattice site (interstitial sites) is called an interstitial defect. This defect associated with the ionic compound is called Frenkel defect or dislocation defect. The ions occupying interstitial sites are called interstitials. The formation of this defect depends on the size of interstitials.
This defect is observed in case of an ionic compound having low coordination number and relatively smaller cations which can fit into interstitial space. This defect is common when the difference in ionic radii of two participating ions is large.
The presence of this defect does not alter the density of the solid. The presence of ions in interstitial sites increases the dielectric constant of the crystal.
Example: In AgCl the defect is observed due to Ag+ ions. In ZnS the defect is observed due to Zn++ ions. AgBr, AgI
Non-Stoichiometric Defects in Crystal Structure:
There are many compounds in which the ratio of positive and negative ions present in the compound differs from that required by the ideal chemical formula of the compound. Such defects are termed as non-stoichiometric or Berthollide compounds. For example, vanadium oxide is represented as VOx. The value of x in the crystal of the oxide varies from 0.6 to 1.3. Similarly, iron oxide is represented as FexO. The value of x in the crystal of the oxide varies from 0.93 to 0.95. The balance of positive and negative charge in such compounds is maintained by having extra electrons or extra positive charges. Such defects are called non-stoichiometric defects.
These defects are of two types: (i) metal excess defect and (ii) metal deficiency defect.
Metal Excess Defect:
A metal excess defect due to anionic vacancies:
This defect is likely to be shown by a crystal which shows Schottky defects. A compound may have excess metal ion if the negative ion is absent from its lattice site, leaving a hole which is occupied by an electron to maintain electrical neutrality.
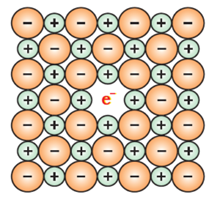
Alkali halides like NaCl and KCl show this type of defect. When crystals of NaCl are heated in an atmosphere of sodium vapour, the sodium atoms are deposited on the surface of the crystal. The Cl– ions diffuse to the surface of the crystal and combine with Na atoms to give NaCl. This happens with the loss of an electron by sodium atoms to form Na+ ions. The released electrons diffuse into the crystal and occupy anionic sites. As a result, the crystal now has an excess of sodium. The anionic sites occupied by unpaired electrons are called F-centres (from the German word Farbenzenter for the colour centre). They impart a yellow colour to the crystals of NaCl. The colour results by excitation of these electrons when they absorb energy from the visible light falling on the crystals. Greater the number of F-centres, greater is the intensity of the colour.
Similarly, the excess of lithium makes LiCl crystals pink and excess of potassium makes KCl crystals violet (or lilac).
Such solids having F- centres are paramagnetic due to the presence of unpaired electrons in the hole.
A Metal excess defect due to the presence of extra cations at interstitial sites:
This defect is due to the presence of extra positive ion at the interstitial site. Electrical neutrality is maintained by the presence of electron at the interstitial site. This defect is likely to be shown by a crystal which shows Frenkel defects.

Zinc oxide is white in colour at room temperature. On heating, it loses oxygen and turns yellow. Now there is an excess of zinc in the crystal and its formula becomes Zn1+xO. The excess Zn2+ ions move to interstitial sites and the electrons to neighbouring interstitial sites. Due to the presence of an electron in interstitial space, the electrical conductivity of ZnO increases on heating.
Metal Deficiency Defect Due to Cationic Vacancies:
The non-stoichiometric compounds may have metal deficiency due to the absence of the metal ion from its lattice site. The electrical neutrality is maintained by acquiring a higher positive charge by the adjacent ion.
There are many solids which are difficult to prepare in the stoichiometric composition and contain less amount of the metal as compared to the stoichiometric proportion.
This type of defect is mainly shown by transition elements. A typical example of this type is FeO which is mostly found with a composition of Fe0.95O. It may actually range from Fe0.93O to Fe0.96O. In crystals of FeO some Fe2+ cations are missing and the loss of positive charge is made up by the presence of the required number of Fe3+ ions.
Note:
Both metal excess compounds and metal-deficient compounds act as semiconductors. Due to the presence of electron metal excess compounds work as n-type semiconductors. While metal deficient compounds conduct electricity through positive hole conduction work as p-type semiconductors.
Point Defects due to Presence of Foreign Atoms or Impurity Defects:
This defect occurs when regular cation of a crystal is replaced by some different cation. The different cation may occupy a regular lattice site or interstitial site.
If the impurity cation is substituted in place of regular cation then the defect is known as substitution impurity defect. If the impurity cation occupies interstitial space then the defect is called interstitial impurity defect.
Interstitial impurity in Stainless Steel:

Substitutional impurity in Brass

Substitutional impurity in NaCl

If molten NaCl containing a little amount of SrCl2 is crystallised, some of the sites of Na+ ions are occupied by Sr2+. Each Sr2+ replaces two Na+ ions. It occupies the site of one ion and the other site remains vacant. The cationic vacancies thus produced are equal in number to that of Sr2+ ions.
Another similar example is the solid solution of CdCl2 and AgCl.
One reply on “Defects in Crystal Structure”
Very good