Science > Chemistry > Solid State > Magnetic Properties of Solids
In this article, we shall study the magnetic properties of solids, classification of solids on the basis of their magnetic properties.
Every substance has some magnetic properties associated with it. The origin of these properties lies in the magnetic moments associated with the electrons. The magnetic moment of electron originates from two types of motions (i) its orbital motion around the nucleus and (ii) its spin around its own axis. Thus electrons behave like tiny magnets. Thus, each electron has a permanent spin and an orbital magnetic moment associated with it.
The magnitude of this magnetic moment is very small and is measured in the unit called Bohr magneton μB. It is equal to 9.27 × 10–24 A m2. The magnitude of the magnetic moment due to spin is ± μB and is directed along the axis of the spin. The magnitude of orbital motion is equal to mlμB and is directed along the axis of rotation. Where ml is the spin quantum number of the electron.
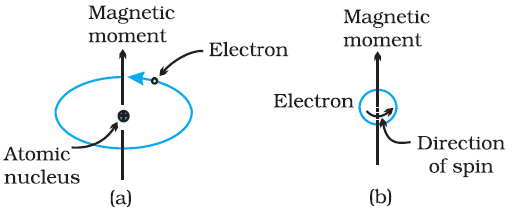
Types of Magnetic Material:
On the basis
of their magnetic properties, substances can be classified into five
categories:
(i) paramagnetic (ii) diamagnetic (iii) ferromagnetic (iv) antiferromagnetic
and (v) ferrimagnetic.
Diamagnetic Substances:
Diamagnetic substances are weakly repelled by a magnetic field. The magnetism exhibited by such substance is called diamagnetism. Examples: H2O, TiO2, V2O5, NaCl and C6H6
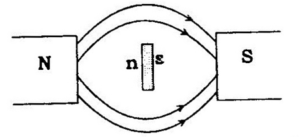
They are weakly magnetized in a magnetic field in opposite direction. Diamagnetism is shown by those substances in which all the electrons are paired and there are no unpaired electrons. The pairing of electrons cancels their magnetic moments and they lose their magnetic character.
Paramagnetic Substances:
Paramagnetic substances are weakly attracted by a magnetic field. Examples: O2, Cu2+, Fe3+, Cr3+, TiO, Ti2O3, VO, VO2, and CuO
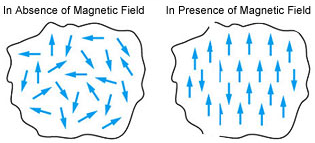
They are magnetized in a magnetic field in the same direction. They lose their magnetism in the absence of a magnetic field. They are temporary magnets. Paramagnetism is due to the presence of one or more unpaired electrons which are attracted by the magnetic field.
Ferromagnetic Substances:
The substances which are attracted very strongly by a magnetic field are called ferromagnetic substances. Examples: iron, cobalt, nickel, gadolinium, and CrO2

Besides strong attractions, these substances can be permanently magnetized. In the solid-state, the metal ions of ferromagnetic substances are grouped together into small regions called domains. Thus, each domain acts as a tiny magnet. In an unmagnetized piece of a ferromagnetic substance, the domains are randomly oriented and their magnetic moments get canceled. When the substance is placed in a magnetic field all the domains get oriented in the direction of the magnetic field and a strong magnetic effect is produced. The alignments of domains persist even when the magnetic field is removed and the ferromagnetic substance becomes a permanent magnet.
Antiferromagnetism:
Substances like MnO showing antiferromagnetism have domain structure similar to ferromagnetic substance, but their domains are oppositely oriented and cancel out each other’s magnetic moment. Examples: V2O3, Cr2O3, MnO, Mn2O3, FeO, Fe2O3, CoO, Co2O3, NiO
Ferrimagnetism:
Ferrimagnetism is observed when the magnetic moments of the domains in the substance are aligned in parallel and anti-parallel directions in unequal numbers. Examples: Fe3O4 (magnetite) and ferrites like MgFe2O4 and ZnFe2O4.
They are weakly attracted by a magnetic field as compared to ferromagnetic substances. These substances also lose ferrimagnetism on heating and become paramagnetic.

Iron is strongly ferromagnetic:
Iron shows strong magnetic properties. It is a ferromagnetic substance and it can be magnetized permanently. The atomic number of iron is 28. Its electronic configuration is [Ar] 3d6 4s2. The box diagram of its electronic configuration is

There are four unpaired electrons. i.e. their spins are not neutralized. Hence they show strong magnetic properties. Hence iron is strongly magnetic.
Guoy’s Method of Studying Magnetic Properties of Solids:

The method consists of weighing the substances in an out of the magnetic field. If the substance is diamagnetic it weighs less in the magnetic field due to the opposite direction of the magnetic field set in the diamagnetic substance (repulsion). If the substance is paramagnetic it weighs more in the magnetic field due to the same direction of magnetic field set in paramagnetic substance (attraction). If the substance is ferromagnetic, then the effect is the same as that in the case of paramagnetic substance but the extent of pull is more than that in the case of paramagnetic substance.