Science > Chemistry > Solid State > Magnetic Properties of Solids
In the last article, we have studied the magnetic properties of solids. In this article, we shall study the dielectric properties of solids.
Source of Dielectric Properties of Solids:
In insulators, electrons in the individual atom or ion are bound to corresponding nuclei. Hence they are not able to migrate. Thus they are localized. Due to this localization, no electrons are available for conduction and insulators do not conduct electricity to them. If the external electric field is applied these atoms or ions undergo polarization due to the formation of dipoles.
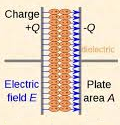
Now the dipoles produced can behave in two ways. They may align themselves in such a way that there is a net dipole moment of the crystal or they may align themselves in such a manner that they cancel each other’s dipole moment and the net dipole moment of the crystal is zero.
Dielectric Properties of Solids:
Due to polarization, the solid show some interesting properties as discussed below.
Piezoelectricity:
The crystal in which the individual dipoles formed due to polarization align themselves in such a way that there is a net dipole moment of the crystal shows piezoelectricity.
When mechanical stress (pressure) is applied to such crystal, the atoms or ions in the crystal are displaced and the crystal produces electricity. Conversely, when an electric field is applied to such crystal, there is displacement 0f the ions or atoms (anti piezoelectricity). Due to these properties, they are used as mechanical-electrical transducers.
They are used in a pickup of record players, pressure sensors, engine knock sensors, etc.
Pyroelectricity:
Some piezoelectric crystals on heating produce electricity. Electricity produced by this method is called pyroelectricity. The phenomenon is called the pyroelectric effect.
They are used in passive infrared (PIR) sensors. They are a common type of motion detector thermal sensors, which can detect the movement of human beings, animals, objects, etc., They are used in infrared non-contact thermometers.
Ferroelectricity:
In some piezoelectric crystals, the dipoles are permanently aligned even in the absence of the electric field. In such crystals, the direction of polarization can be shifted by applying an external electrical field. This phenomenon is known as ferroelectricity.
They are used in capacitors as a dielectric, in non-volatile memory, in ultrasound imaging and actuators, for making thermistors, oscillators, filters, light deflectors, modulators, and displays.
Examples of such materials are Barium Titanate (BaTiO3), Lead Titanate (PbTiO3), sodium potassium tartarate (Rochelle salt), Potassium dihydrogen phosphate (KH2PO4), etc.
Anti-ferroelectricity:
When the dipole in alternate polyhedra point up and down, the net dipole moment of the crystal is zero. Such a crystal is called antiferroelectric.
Example – Lead zirconate (PbZrO3)