Science > Chemistry > Concept of Atomic Mass and Equivalent Mass > Atomic Mass by Dulong Petit’s Law
In previous articles, we have studied Cannizzaro’s method and law of isomorphism method to determine atomic mass. In this article, we shall study to calculate atomic mass by Dulong Petit’s law.

Specific Heat:
The amount of heat required to raise the temperature of one mole of an element from 287.5 K to 288.5 K is called specific heat of the element. Its S.I. unit is J/mol/ K.
The product of atomic mass and specific heat of the element is called atomic heat of the element.
Dulong-Petit’s Law:
The product of specific heat and the atomic mass of an element in the solid-state is approximately equal to 6.4. OR Atomic heat of a solid element is nearly equal to 6.4.
Limitations of Dulong-Petit’s Law:
- This law is applicable to elements which are in solid state.
- This law Is applicable to the heavier element. It is not applicable to lighter elements having high melting points.
- This law gives approximate atomic mass.
Steps Involved:
- Find the equivalent mass of the element by any method mentioned in topic equivalent mass.
- Find approximate atomic mass using relation, Approx. atomic mass × Specific heat = 6.4
- Find the valency of the element using relation, Approx. atomic mass = equivalent mass × valency
- Find the nearest whole number for the calculated valency and use this whole number as valency of that element.
- Use following formula to calculate the corrected atomic mass of the element, Corrected atomic mass = Equivalent mass × valency
Numerical Methods:
Example – 01:
The specific heat of a metal A is found to be 0.03; its equivalent mass is 69.66. Calculate the valency and exact atomic mass of an element.
Solution:
By Dulong-Petit’s Law, Atomic mass × Specific heat = 6.4 (Approx.)
∴ Approx. atomic mass = 6.4 / Sp. heat = 6.4 /0.03 = 213.33
Now, approx. atomic mass = equivalent mass × valency
∴ Valency = approx. atomic mass / equivalent mass = 213.33 / 69.66 = 3.06
∴ Valency = 3 (Corrected to nearest whole number)
Now, corrected atomic mass = Equivalent mass × valency = 69.66 × 3 = 208.98 u
Ans: Hence the valency is 3 and its atomic mass is 208.98 u.
Example – 02:
A solid element of equivalent mass 9 has specific heat 1 J/g/K. calculate its atomic mass.
Solution:
Specific heat = 1 J/g/K = 1 / 4.188 = 0.2388
By Dulong-Petit’s Law, Atomic mass × Specific heat = 6.4 (Approx.)
∴ Approx. atomic mass = 6.4 / Sp. heat = 6.4 /0.2388 = 26.80
Now, approx. atomic mass = equivalent mass × valency
∴ Valency = approx. atomic mass / equivalent mass = 26.80 / 9 = 2.98
∴ Valency = 3 (Corrected to nearest whole number)
Now, corrected atomic mass = Equivalent mass × valency = 9 × 3 = 27 u
Ans: Hence the valency is 3 and its atomic mass is 27 u.
Example – 03:
Equivalent mass of barium is 68.68 and its valency is 2. Find its atomic mass.
Solution:
Equivalent mass of barium = 68.68, valency of barium = 2
Atomic mass = Equivalent mass x valency = 68.68 x 2 = 137.6
Thus atomic mass of barium is 137.6.
Example – 04:
The specific heat of a metal was found to be 0.03 and its equivalent mass is 103.5. Find the atomic mass of the metal.
Solution:
By Dulong-Petit’s Law, Atomic mass × Specific heat = 6.4 (Approx.)
∴ Approx. atomic mass = 6.4 / Sp. heat = 6.4 /0.03 = 213.33
Now, approx. atomic mass = equivalent mass × valency
∴ Valency = approx. atomic mass / equivalent mass = 213.33 / 103.5 = 2.05
∴ Valency = 2 (Corrected to nearest whole number)
Now, Corrected atomic mass = Equivalent mass × valency = 103.5 × 2 = 207 u
Ans: atomic mass is 207 u.
Example – 05:
The specific heat of metal M that forms sulphide MS is 0.032 cal g-1 deg-1. What is the equivalent mass of the metal?
Solution:
By Dulong-Petit’s Law, Atomic mass × Specific heat = 6.4 (Approx.)
∴ Approx. atomic mass = 6.4 / Sp. heat = 6.4 /0.032 = 200
Now metal sulphide has formula MS
Hence valency of metal is 2
Now, approx. atomic mass = equivalent mass × valency
∴ Equivalent mass = approx. atomic mass / valency = 200 / 2 = 100
Ans: equivalent mass is 100 u.
Example – 06:
The chloride of a metal was found to contain 26.2 % of chlorine. The specific heat of the metal is 0.033. Calculate the atomic mass and equivalent mass of the metal. Also write the molecular formula of metal chloride.
Solution:
Consider 100 g of metal chloride
% of chlorine = 26.2
% of metal = 100 – 26.2 = 73.8
Mass of chlorine = 26.2 g, Mass of metal = 73.8 g
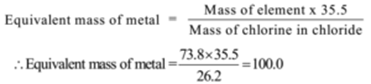
By Dulong Petit’s Law, Atomic mass × Specific heat = 6.4 (Approx.)
∴ Approx. atomic mass = 6.4 / Sp. heat = 6.4 /0.033 = 194
Now, approx. atomic mass = equivalent mass × valency
∴ Valency = approx. atomic mass / equivalent mass = 194 / 100 = 1.94
∴ Valency = 2 (Corrected to nearest whole number)
Now, Corrected atomic mass = Equivalent mass × valency = 100 × 2 = 200 u
Valency of metal (M) is 2 and that of chlorine is 1. Hence the formula of metal chloride is MCl2.
Ans: the atomic mass of the metal is 200 and its equivalent mass is 100. The formula of metal chloride is MCl2.
Example – 07:
1 g of metal having specific heat 0.060205 combines with oxygen to form 1.08 g of oxide. Find atomic mass and valency of the metal. Also, write the molecular formula of the metal oxide.
Solution:
Mass of metal = 1 g, Mass of oxide = 1.08 g
Mass of oxygen = 1.08 g – 1 g = 0.08 g

By Dulong Petit’s Law, Atomic mass × Specific heat = 6.4 (Approx.)
∴ Approx. atomic mass = 6.4 / Sp. heat = 6.4 /0.060205 = 103.1
Now, approx. atomic mass = equivalent mass × valency
∴ Valency = approx. atomic mass / equivalent mass = 103.1 / 100 = 1.03
∴ Valency = 1 (Corrected to nearest whole number)
Now, Corrected atomic mass = Equivalent mass × valency = 100 × 1 = 200 u
Valency of metal (M) is 1 and that of oxygen is 2. Hence the formula of metal chloride is M2O.
Ans: the atomic mass of the metal is 100 and its valency is 1, the formula of metal chloride is M2O.
Example – 08:
A metallic oxide contains 47.06 % of oxygen. The specific heat of metal is 0.25. Calculate the atomic mass of the metal. Write molecular formula of metal oxide.
Solution:
Consider 100 g of metal oxide
% of oxygen = 47.06
% of metal = 100 – 47.06 = 52.94
Mass of oxygen = 47.06 g, Mass of metal = 52.94 g

Equivalent mass = (52.94 × 8) / 47.06 = 9
By Dulong Petit’s Law, Atomic mass × Specific heat = 6.4 (Approx.)
∴ Approx. atomic mass = 6.4 / Sp. heat = 6.4 /0.25 = 25.6
Now, approx. atomic mass = equivalent mass × valency
∴ Valency = approx. atomic mass / equivalent mass = 25.6 / 9 = 2.8
∴ Valency = 3 (Corrected to nearest whole number)
Now, Corrected atomic mass = Equivalent mass × valency = 9 × 3 = 27 u
Valency of metal (M) is 3 and that of oxygen is 2. Hence the formula of metal chloride is M2O3.
Ans: the atomic mass of the metal is 27 and the formula of metal oxide is M2O3.
Example – 09:
0.54 g of a metal combines with 0.48 g of oxygen to form its oxide. Its specific heat is 0.22 cal per deg. What is the atomic mass of the metal?
Solution:
Mass of oxygen = 0.48 g, Mass of metal = 0.54 g

Equivalent mass = (0.54 × 8) / 0.48 = 9
By Dulong Petit’s Law, Atomic mass × Specific heat = 6.4 (Approx.)
∴ Approx. atomic mass = 6.4 / Sp. heat = 6.4 /0.22 = 29.09
Now, approx. atomic mass = equivalent mass × valency
∴ Valency = approx. atomic mass / equivalent mass = 29.09 / 9 = 3.2
∴ Valency = 3 (Corrected to nearest whole number)
Now, Corrected atomic mass = Equivalent mass × valency = 9 × 3 = 27 u
Ans: the atomic mass of the metal is 27.
Example – 10:
0.45 g of metal displaced 560 ml of hydrogen at STP from acid. Specific heat of metal is 0.214. Calculate the equivalent mass and atomic mass of the metal.
Solution:
Mass of metal = 0.45 g
Volume of hydrogen = 560 ml = 0.560 dm³ at STP
Mass of 0.560 dm³ of hydrogen at STP
= (Molecular mass of gas x volume of gas in dm³) / 22.4
Mass of hydrogen displaced = (2 x 0.560) / 22.4 = 0.05 g

Equivalent mass of metal = 0.45 / 0.05 = 9
By Dulong Petit’s Law, Atomic mass × Specific heat = 6.4 (Approx.)
∴ Approx. atomic mass = 6.4 / Sp. heat = 6.4 /0.214 = 29.9
Now, approx. atomic mass = equivalent mass × valency
Now, approx. atomic mass = equivalent mass × valency
∴ Valency = approx. atomic mass / equivalent mass = 29.9 / 9 = 3.3
∴ Valency = 3 (Corrected to nearest whole number)
Now, Corrected atomic mass = Equivalent mass × valency = 9 × 3 = 27 u
Ans: the atomic mass of the metal is 27 and its equivalent mass is 9.
Example – 11:
0.2160 g of metal, when treated with an excess of dilute sulphuric acid gave 80.4 cc of moist hydrogen measured at 20 °C and 770 mm of pressure. The specific heat of the metal is 0.0955. Calculate the valency and exact atomic mass of the metal. The aqueous tension at 20 °C is 17.5 mm.
Solution:

By Dulong Petit’s Law, Atomic mass × Specific heat = 6.4 (Approx.)
∴ Approx. atomic mass = 6.4 / Sp. heat = 6.4 /0.0955 = 67.02
Now, approx. atomic mass = equivalent mass × valency
Now, approx. atomic mass = equivalent mass × valency
∴ Valency = approx. atomic mass / equivalent mass = 67.02 / 32.36 = 2.07
∴ Valency = 2 (Corrected to nearest whole number)
Now, Corrected atomic mass = Equivalent mass × valency
= 32.36 × 2 = 64.72 u
Ans: the atomic mass of the metal is 64.72 and its valency is 2.
Example – 12:
0.45 g of metal gave 176.6 ml of hydrogen at 23 °C and 743 mm pressure when treated with dilute sulphuric acid. Calculate the equivalent mass of the metal. Aqueous tension at 23 °C is 21 mm. If the specific heat of the metal is 0.091, calculate the exact atomic mass of the metal.
Solution:

By Dulong Petit’s Law, Atomic mass × Specific heat = 6.4 (Approx.)
∴ Approx. atomic mass = 6.4 / Sp. heat = 6.4 /0.091 = 70.33
Now, approx. atomic mass = equivalent mass × valency
∴ Valency = approx. atomic mass / equivalent mass = 70.33 / 32.32 = 2.1
∴ Valency = 2 (Corrected to nearest whole number)
Now, Corrected atomic mass = Equivalent mass × valency = 32.32 × 2 = 64.64 u
Ans: the atomic mass of the metal is 64.64 and its valency is 2.
Example – 13:
A metal M forms a volatile chloride, containing 80% of chlorine. The vapour density of the chloride is 66.75. Calculate the exact atomic mass of the element.
Solution:
Molecular mass = 2 x Vapour Density = 2 x 66.75 = 133.5
% of chlorine = 80
% of the element = 100 – 80 = 20
Mass of element = 20 g, Mass of chlorine = 80 g

Equivalent mass of metal = (20 x 35.5) / 80 = 8.875
Let valency of the metal be ‘x’, hence the formula of the chloride is MClx.
Atomic mass of metal = Equivalent mass x valency = 8.875 x
Molecular mass of chloride = Atomic mass of metal + Atomic mass of chlorine × x
Molecular mass of chloride = 8.875 x + 35.5 × x = 133.5
∴ 8.875 x + 35.5 × x = 133.5
∴ 44.375 x = 133.5
∴ x = 133.5 /44.375 = 3.01
∴ Valency = 3 (Corrected to nearest whole number)
Actual atomic mass = Equivalent mass x valency = 8.875 x 3 = 26.625
Ans: The exact atomic mass of the element is 26.635
Example – 14:
The specific heat of a metal A is found to be 0.03. 10 g of metal on evaporation of nitric acid gave 18.9 g of pure dry nitrate. Calculate the equivalent mass and exact atomic mass of the metal.
Solution:
Mass of metal = 10 g
By double decomposition method

By Dulong Petit’s Law, Atomic mass × Specific heat = 6.4 (Approx.)
∴ Approx. atomic mass = 6.4 / Sp. heat = 6.4 /0.03 = 213.33
Now, approx. atomic mass = equivalent mass × valency
∴ Valency = approx. atomic mass / equivalent mass = 213.33 / 69.66 = 3.06
∴ Valency = 3 (Corrected to nearest whole number)
Now, Corrected atomic mass = Equivalent mass × valency = 69.66 × 3 = 208.98 u
Ans: the atomic mass of the metal is 208.98 and its equivalent mass is 69.66.
Example – 15:
1 g of metallic bromide dissolved in water gave with the excess of silver nitrate, 1.88 g of silver bromide. Calculate the accurate atomic mass of the element, if its specific heat is 0.15 cal (atomic mass of Ag is 108 and that of bromine is 80).
Solution:
Molecular mass of silver bromide = 108 + 80 = 188 g
Mass of bromine in 1.88 g of silver bromide = (80/180) x 1.8 = 0.8 g
Mass of metallic bromide = 1g, Mass of bromine = 0.8 g
Mass of metal = 1 – 0.8 = 0.2 g
By double decomposition method

By Dulong Petit’s Law, Atomic mass × Specific heat = 6.4 (Approx.)
∴ Approx. atomic mass = 6.4 / Sp. heat = 6.4 /0.15 = 42.67
Now, approx. atomic mass = equivalent mass × valency
∴ Valency = approx. atomic mass / equivalent mass = 42.67 / 20 = 2.1
∴ Valency = 2 (Corrected to nearest whole number)
Now, Corrected atomic mass = Equivalent mass × valency = 20 × 2 =40 u
Ans: the atomic mass of the metal is 40.
In next article, we shall study the concept of equivalent mass and hydrogen displacement method to determine it.
Previous Topic: Atomic Mass Using Law of Isomorphism
Next Topic: Equivalent Mass by Hydrogen Displacement Method