Science > Chemistry > Electrochemistry > Electrolysis and its Applications
A process in which electrical energy is converted into chemical energy by using a suitable device known as an electrolytic cell is called electrolysis.
Electrolysis of Fused NaCl:
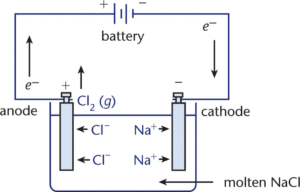
Construction of Electronic Cell or Electrolytic Cell:
The electrolytic cell consists of a vessel made up of a non-conducting material like glass and of a suitable size. The vessel contains an electrolyte to be electrolyzed (fused NaCl). An electrolyte is a substance which in its aqueous solution or fused state liberates ions and allows the electric current to pass through it.
Two electrodes, cathode, and anode are dipped in the electrolyte. The electrodes are metallic or non-metallic plates or rods which conducts electric current. A dry cell or lead accumulator or rectifier is used as a source of direct current. The electrode which is connected to the negative terminal of the battery is called the cathode and the electrode connected to the positive terminal of the battery is called the anode. Cathode and anode are connected externally through a battery.
Working:
When electrical energy passes through the electrolyte, ions of the solution move to oppositely charged electrodes and are discharged. Positive ions move to the cathode while negative ions move to the anode. In this way, different products are either liberated or deposited at respective electrodes.
Reactions:
NaCl is decomposed by passing electrical energy through fused NaCl.
Dissociation of NaCl
NaCl → Na+ + Cl–
Reduction half-reaction at the cathode:
Na+ ions move to the cathode and gain an electron and are reduced.
Na+(l) + 1 e– → Na(s) (reduction)
Reaction taking place at the cathode is called cathodic reduction or electronation.
Oxidation half-reaction at the anode:
Cl– ions move to the anode and lose electrons and are oxidised.
Cl– (l) → Cl(g) + 1 e– (oxidation)
Cl(g) + Cl(g) → Cl2 (g)
Reaction taking place at anode is called anodic oxidation or de-electronation.
Overall (net) cell reaction :
2 Na+(l) + 2Cl–(l) → 2 Na(s) + Cl2(g)
In this way, the molten silvery-white Na is deposited at the cathode and pale green chlorine gas is liberated at the anode.
Electrolysis of aqueous NaCl:
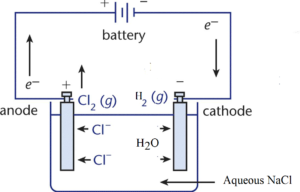
The electrolysis of aqueous NaCl can be carried out by a similar arrangement as that for electrolysis of molten NaCl. Due to the involvement of water, the reactions taking place at electrodes are different.
Reduction half-reaction at the cathode:
Na+(l) + 1 e– → Na(s) (reduction) and
2H2O + 2e– → H2↑ + 2OH–(aq)
The standard reduction potential of water is higher than that for sodium ion. Thus water has a much great tendency to get reduced than sodium ion. Hence water gets reduced at the cathode.
Oxidation half-reaction at the anode:
Cl–(aq) → Cl(g) + 1 e– (oxidation)
Cl(g) + Cl(g) → Cl2(g)
2H2O(l) → O2(g) + 4H+(aq) + 4e–
The standard oxidation potential of water is higher than that for chlorine ion. Thus water has a much great tendency to get oxidized than chlorine ion. Hence it is expected that the cathode half-reaction must be oxidation of water to O2 gas. However, the experimental observation shows that the gas produced at the anode is Cl2 and not O2 gas. That means there is an oxidation of Cl– to Cl2. It is due to the phenomenon called overvoltage.
Overall (Net) Reaction:
Cl–(aq) + 2H2O(l) → Cl2(g) + H2(g) + 2OH–(aq)
Thus H2 gas is liberated at the cathode and Cl2 gas is liberated at the anode. Na+ ions in solution react with OH- ions in solution to form NaOH. Thus NaCl solution gets converted into NaOH.
Concept of Overvoltage:
In electrolysis of aqueous NaCl, it is expected that the cathode half-reaction must be oxidation of water to O2 gas. However, the experimental observation shows that the gas produced at the anode is Cl2 and not O2 gas. That means there is an oxidation of Cl- to Cl2 . It is due to a phenomenon called overvoltage.
It has been found experimentally that the actual voltage required for electrolysis is greater than that calculated from standard potentials. This additional voltage required is the overvoltage. This additional voltage is required because the rate of transfer of electrons at the interface of the electrode and solution for both the half-reaction is slow. The overvoltage for the formation of O2 is much larger than that required for the formation of Cl2. Hence Cl2 is liberated at the anode.
Electrolysis of fused MgCl2:

Reactions:
Fused MgCl2 is decomposed by passing electrical energy through it.
Dissociation of MgCl2
MgCl2(l) → Mg++(l) + 2Cl– (l)
Reduction half-reaction at the cathode: Mg++ ions move to the cathode and gain electrons and are reduced.
Mg++(l) + 2 e– → Mg(l) (reduction)
Reaction taking place at the cathode is called cathodic reduction or electronation.
Oxidation half-reaction at the anode: Cl– ions move to the anode and lose electrons and are oxidised.
2Cl– (l) → Cl2(g) + 2 e– (oxidation)
The reaction taking place at the anode is called anodic oxidation or de-electronation.
Overall (net) cell reaction :
Mg++(l) + 2Cl– (l) → Mg(l) + Cl2(g)
In this way, molten Mg is deposited at cathode and pale green chlorine gas is liberated at anode.
Applications of Electrolysis:
Determination of Equivalent Masses of Elements:
According to the second law of electrolysis when the same quantity of electronic current is passed through solutions of salts of two different cells, the amounts of the metals deposited on the cathodes of the two cells are proportional to their equivalent masses of the respective metals. If the amounts of the metals deposited on the cathodes be W1 and W2 respectively, then

Knowing the equivalent mass of one metal, the equivalent mass of the other metal can be calculated from the above relationship. The equivalent masses of those non-metals which are evolved at anodes can also be determined by this method.
Electrometallurgy:
Electrometallurgy is the process of extraction of metal from ore by electrolysis. The metals like sodium, potassium, magnesium, calcium aluminum, etc., are obtained by electrolysis of fused electrolytes.


Manufacture of Non-Metals:
- Non-metals like hydrogen, fluorine, chlorine are obtained by electrolysis.
- Fluorine is prepared from KHF2 using copper as a reaction vessel and graphite as an electrode.
Electrorefining:
This is the process of refining the metal i.e. removing impurity from metal using electrolysis. The metals like copper, silver, gold, aluminum, tin, etc., are refined by electrolysis. Generally, the impure metal rod is used as the anode and the pure metal rod is used as the cathode. Electrolytic solution used contain electrolyte containing the metal ion to be refined.

Electroplating:
The process of coating an inferior metal with a superior metal by electrolysis is known as electroplating. The aims of electroplating are:
- To prevent the inferior metal from corrosion.
- To make it more attractive in appearance.
The object to be electroplated is made the cathode and block of the metal to be deposited is made the anode in an electrolytic bath containing a solution of a salt of the anodic metal. On passing electric current in the cell, the metal of the anode dissolves out and is deposited on the cathode-article in the form of a thin film.

The following are the requirements for fine coating:
- The surface of the article should be free from greasy matter and its oxide layer. The surface is cleaned with chromic acid or detergents.
- The surface of the article should be rough so that the metal deposited sticks permanently.
- The concentration of the electrolyte should be so adjusted as to get a smooth coating.
- The current density must be the same throughout.
Previous Topic: types of Cells
Next Topic: Electrochemical Cells (Primary Cells)